Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry
- PMID: 15113897
- PMCID: PMC400355
- DOI: 10.1128/jvi.78.10.5147-5156.2004
Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry
Abstract
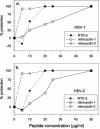
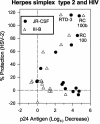
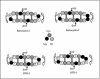
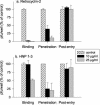
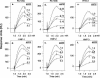
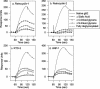
We tested the ability of 20 synthetic theta defensins to protect cells from infection by type 1 and type 2 herpes simplex viruses (HSV-1 and -2, respectively). The peptides included rhesus theta defensins (RTDs) 1 to 3, originally isolated from rhesus macaque leukocytes, and three peptides (retrocyclins 1 to 3) whose sequences were inferred from human theta-defensin (DEFT) pseudogenes. We also tested 14 retrocyclin analogues, including the retro, enantio, and retroenantio forms of retrocyclin 1. Retrocyclins 1 and 2 and RTD 3 protected cervical epithelial cells from infection by both HSV serotypes, but only retrocyclin 2 did so without causing cytotoxicity or requiring preincubation with the virus. Surface plasmon resonance studies revealed that retrocyclin 2 bound to immobilized HSV-2 glycoprotein B (gB2) with high affinity (K(d), 13.3 nM) and that it did not bind to enzymatically deglycosylated gB2. Temperature shift experiments indicated that retrocyclin 2 and human alpha defensins human neutrophil peptide 1 (HNP 1) to HNP 3 protected human cells from HSV-2 by different mechanisms. Retrocyclin 2 blocked viral attachment, and its addition during the binding or penetration phases of HSV-2 infection markedly diminished nuclear translocation of VP16 and expression of ICP4. In contrast, HNPs 1 to 3 had little effect on binding but reduced both VP16 transport and ICP4 expression if added during the postbinding (penetration) period. We recently reported that theta defensins are miniature lectins that bind gp120 of human immunodeficiency virus type 1 (HIV-1) with high affinity and inhibit the entry of R5 and X4 isolates of HIV-1. Given its small size (18 residues), minimal cytotoxicity, lack of activity against vaginal lactobacilli, and effectiveness against both HSV-2 and HIV-1, retrocyclin 2 provides an intriguing prototype for future topical microbicide development.
Figures




Similar articles
-
RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates.AIDS Res Hum Retroviruses. 2004 Nov;20(11):1157-65. doi: 10.1089/aid.2004.20.1157. AIDS Res Hum Retroviruses. 2004. PMID: 15588337
-
Activity of alpha- and theta-defensins against primary isolates of HIV-1.J Immunol. 2004 Jul 1;173(1):515-20. doi: 10.4049/jimmunol.173.1.515. J Immunol. 2004. PMID: 15210812
-
Retrocyclin, an antiretroviral theta-defensin, is a lectin.J Immunol. 2003 May 1;170(9):4708-16. doi: 10.4049/jimmunol.170.9.4708. J Immunol. 2003. PMID: 12707350
-
Retrocyclins: using past as prologue.Curr Protein Pept Sci. 2004 Oct;5(5):373-81. doi: 10.2174/1389203043379657. Curr Protein Pept Sci. 2004. PMID: 15544532 Review.
-
Theta-defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins.Curr Protein Pept Sci. 2004 Oct;5(5):365-71. doi: 10.2174/1389203043379459. Curr Protein Pept Sci. 2004. PMID: 15544531 Review.
Cited by
-
Meclizine Inhibits Pseudorabies Virus Replication by Interfering With Virus Entry and Release.Front Microbiol. 2021 Dec 22;12:795593. doi: 10.3389/fmicb.2021.795593. eCollection 2021. Front Microbiol. 2021. PMID: 35003025 Free PMC article.
-
Resistance is futile: targeting multidrug-resistant bacteria with de novo Cys-rich cyclic polypeptides.RSC Chem Biol. 2023 Aug 21;4(10):722-735. doi: 10.1039/d3cb00015j. eCollection 2023 Oct 4. RSC Chem Biol. 2023. PMID: 37799576 Free PMC article. Review.
-
Structure and Biological Functions of β-Hairpin Antimicrobial Peptides.Acta Naturae. 2015 Jan-Mar;7(1):37-47. Acta Naturae. 2015. PMID: 25927000 Free PMC article.
-
Antiviral strategies targeting herpesviruses.J Virus Erad. 2021 May 30;7(3):100047. doi: 10.1016/j.jve.2021.100047. eCollection 2021 Sep. J Virus Erad. 2021. PMID: 34141443 Free PMC article. Review.
-
Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells.J Virol. 2007 Apr;81(8):3731-9. doi: 10.1128/JVI.02250-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229687 Free PMC article.
References
-
- Barrientos, L. G., B. R. O'Keefe, M. Bray, A. Sanchez, A. M. Gronenborn, and M. R. Boyd. 2003. Cyanovirin-N binds to the viral surface glycoprotein, GP(1,2) and inhibits infectivity of Ebola virus. Antivir. Res. 58:47-56. - PubMed
-
- Barry, D. G., N. L. Daly, R. J. Clark, L. Sando, and D. J. Craik. 2003. Linearization of a naturally occurring circular protein maintains structure but eliminates hemolytic activity. Biochemistry 42:6688-6695. - PubMed
-
- Bastian, A., and H. Schafer. 2001. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 101:157-161. - PubMed
-
- Bax, R., K. Douville, D. McCormick, M. Rosenberg, J. Higgins, and M. Bowden. 2002. Microbicides—evaluating multiple formulations of C31G. Contraception 66:365-368. - PubMed
-
- Beutler, J. A., J. B. McMahon, T. R. Johnson, B. R. O'Keefe, R. A. Buzzell, D. Robbins, R. Gardella, J. Wilson, and M. R. Boyd. 2002. High throughput screening for cyanovirin-N mimetics binding to HIV-1 gp41. J. Biomol. Screen. 7:105-110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

