CD4-induced T-20 binding to human immunodeficiency virus type 1 gp120 blocks interaction with the CXCR4 coreceptor
- PMID: 15113923
- PMCID: PMC400340
- DOI: 10.1128/jvi.78.10.5448-5457.2004
CD4-induced T-20 binding to human immunodeficiency virus type 1 gp120 blocks interaction with the CXCR4 coreceptor
Abstract
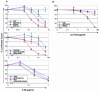
The synthetic peptide T-20, which corresponds to a sequence within the C-terminal heptad repeat region (HR2) of the human immunodeficiency virus type 1 (HIV-1) gp41 envelope glycoprotein, potently inhibits viral membrane fusion and entry. Although T-20 is thought to bind the N-terminal heptad repeat region (HR1) of gp41 and interfere with gp41 conformational changes required for membrane fusion, coreceptor specificity determined by the V3 loop of gp120 strongly influences the sensitivity of HIV-1 variants to T-20. Here, we show that T-20 binds to the gp120 glycoproteins of HIV-1 isolates that utilize CXCR4 as a coreceptor in a manner determined by the sequences of the gp120 V3 loop. T-20 binding to gp120 was enhanced in the presence of soluble CD4. Analysis of T-20 binding to gp120 mutants with variable loop deletions and the reciprocal competition of T-20 and particular anti-gp120 antibodies suggested that T-20 interacts with a gp120 region near the base of the V3 loop. Consistent with the involvement of this region in coreceptor binding, T-20 was able to block the interaction of gp120-CD4 complexes with the CXCR4 coreceptor. These results help to explain the increased sensitivity of CXCR4-specific HIV-1 isolates to the T-20 peptide. Interactions between the gp41 HR2 region and coreceptor-binding regions of gp120 may also play a role in the function of the HIV-1 envelope glycoproteins.
Figures
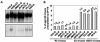



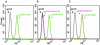

Similar articles
-
Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor.J Virol. 2001 Sep;75(18):8605-14. doi: 10.1128/jvi.75.18.8605-8614.2001. J Virol. 2001. PMID: 11507206 Free PMC article.
-
Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120.J Virol. 2000 Sep;74(18):8358-67. doi: 10.1128/jvi.74.18.8358-8367.2000. J Virol. 2000. PMID: 10954535 Free PMC article.
-
Mutations That Increase the Stability of the Postfusion gp41 Conformation of the HIV-1 Envelope Glycoprotein Are Selected by both an X4 and R5 HIV-1 Virus To Escape Fusion Inhibitors Corresponding to Heptad Repeat 1 of gp41, but the gp120 Adaptive Mutations Differ between the Two Viruses.J Virol. 2019 May 15;93(11):e00142-19. doi: 10.1128/JVI.00142-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894471 Free PMC article.
-
CD4 activation of HIV fusion.Int J Cell Cloning. 1992 Nov;10(6):323-32. doi: 10.1002/stem.5530100603. Int J Cell Cloning. 1992. PMID: 1281202 Review.
-
Development of anti-HIV agents targeting dynamic supramolecular mechanism: entry and fusion inhibitors based on CXCR4/CCR5 antagonists and gp41-C34-remodeling peptides.Curr HIV Res. 2005 Oct;3(4):289-301. doi: 10.2174/157016205774370429. Curr HIV Res. 2005. PMID: 16250877 Review.
Cited by
-
Inhibition of envelope-mediated CD4+-T-cell depletion by human immunodeficiency virus attachment inhibitors.Antimicrob Agents Chemother. 2009 Nov;53(11):4726-32. doi: 10.1128/AAC.00494-09. Epub 2009 Aug 31. Antimicrob Agents Chemother. 2009. PMID: 19721067 Free PMC article.
-
Single-molecule analysis of human immunodeficiency virus type 1 gp120-receptor interactions in living cells.J Virol. 2005 Dec;79(23):14748-55. doi: 10.1128/JVI.79.23.14748-14755.2005. J Virol. 2005. PMID: 16282475 Free PMC article.
-
Selection with a peptide fusion inhibitor corresponding to the first heptad repeat of HIV-1 gp41 identifies two genetic pathways conferring cross-resistance to peptide fusion inhibitors corresponding to the first and second heptad repeats (HR1 and HR2) of gp41.J Virol. 2011 Dec;85(24):12929-38. doi: 10.1128/JVI.05391-11. Epub 2011 Oct 12. J Virol. 2011. PMID: 21994458 Free PMC article.
-
The six-helix bundle of human immunodeficiency virus Env controls pore formation and enlargement and is initiated at residues proximal to the hairpin turn.J Virol. 2009 Oct;83(19):10048-57. doi: 10.1128/JVI.00316-09. Epub 2009 Jul 22. J Virol. 2009. PMID: 19625396 Free PMC article.
-
Peptides in the treatment of AIDS.Curr Opin Struct Biol. 2009 Aug;19(4):473-82. doi: 10.1016/j.sbi.2009.07.003. Epub 2009 Jul 23. Curr Opin Struct Biol. 2009. PMID: 19632107 Free PMC article. Review.
References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Babcock, G. J., T. Mirzabekov, W. Wojtowicz, and J. Sodroski. 2001. Ligand binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes. J. Biol. Chem. 276:38433-38440. - PubMed
-
- Bandres, J. C., Q. F. Wang, J. O'Leary, F. Baleaux, A. Amara, J. A. Hoxie, S. Zolla-Pazner, and M. K. Gorny. 1998. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J. Virol. 72:2500-2504. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

