Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells
- PMID: 15115287
- PMCID: PMC514906
- DOI: 10.1379/1466-1268(2003)008<0348:hspria>2.0.co;2
Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells
Abstract
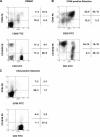
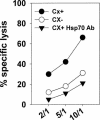
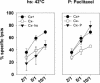
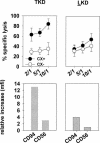
Previously we described an involvement of the C-type lectin receptor CD94 and the neuronal adhesion molecule CD56 in the interaction of natural killer (NK) cells with Hsp70-protein and Hsp70-peptide TKD. Therefore, differences in the cell surface density of these NK cell-specific markers were investigated comparatively in CD94-sorted, primary NK cells and in established NK cell lines NK-92, NKL, and YT after TKD stimulation. Initially, all NK cell types were positive for CD94; the CD56 expression varied. After stimulation with TKD, the mean fluorescence intensity (mfi) of CD94 and CD56 was upregulated selectively in primary NK cells but not in NK cell lines. Other cell surface markers including natural cytotoxicity receptors remained unaffected in all cell types. CD3-enriched T cells neither expressing CD94 nor CD56 served as a negative control. High receptor densities of CD94/CD56 were associated with an increased cytolytic response against Hsp70 membrane-positive tumor target cells. The major histocompatibility complex (MHC) class I-negative, Hsp70-positive target cell line K562 was efficiently lysed by primary NK cells and to a lower extent by NK lines NK-92 and NKL. YT and CD3-positive T cells were unable to kill K562 cells. MHC class-I and Hsp70-positive, Cx + tumor target cells were efficiently lysed only by CD94-sorted, TKD-stimulated NK cells with high CD94/CD56 mfi values. Hsp70-specificity was demonstrated by antibody blocking assays, comparative phenotyping of the tumor target cells, and by correlating the amount of membrane-bound Hsp70 with the sensitivity to lysis. Remarkably, a 14-mer peptide (LKD), exhibiting only 1 amino acid exchange at position 1 (T to L), neither stimulated Hsp70-reactivity nor resulted in an upregulated CD94 expression on primary NK cells. Taken together our findings indicate that an MHC class I-independent, Hsp70 reactivity could be associated with elevated cell surface densities of CD94 and CD56 after TKD stimulation.
Figures


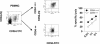
Similar articles
-
The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells.J Immunol. 2004 Jan 15;172(2):972-80. doi: 10.4049/jimmunol.172.2.972. J Immunol. 2004. PMID: 14707070
-
Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94.Biol Chem. 2003 Feb;384(2):267-79. doi: 10.1515/BC.2003.030. Biol Chem. 2003. PMID: 12675520
-
Inhibition of tumor growth in mice with severe combined immunodeficiency is mediated by heat shock protein 70 (Hsp70)-peptide-activated, CD94 positive natural killer cells.Cell Stress Chaperones. 2002 Oct;7(4):365-73. doi: 10.1379/1466-1268(2002)007<0365:IOTGIM>2.0.CO;2. Cell Stress Chaperones. 2002. PMID: 12653481 Free PMC article.
-
The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor.Mol Immunol. 2005 Feb;42(4):485-8. doi: 10.1016/j.molimm.2004.07.031. Mol Immunol. 2005. PMID: 15607803 Review.
-
The role of natural killer cells in viral infections.Scand J Immunol. 1997 Sep;46(3):217-24. doi: 10.1046/j.1365-3083.1997.d01-121.x. Scand J Immunol. 1997. PMID: 9315107 Review.
Cited by
-
Biological properties of extracellular vesicles and their physiological functions.J Extracell Vesicles. 2015 May 14;4:27066. doi: 10.3402/jev.v4.27066. eCollection 2015. J Extracell Vesicles. 2015. PMID: 25979354 Free PMC article.
-
The endogenous danger signals HSP70 and MICA cooperate in the activation of cytotoxic effector functions of NK cells.J Cell Mol Med. 2010 Apr;14(4):992-1002. doi: 10.1111/j.1582-4934.2009.00677.x. J Cell Mol Med. 2010. PMID: 20569278 Free PMC article.
-
Targeted Ferroptosis-Immunotherapy Synergy: Enhanced Antiglioma Efficacy with Hybrid Nanovesicles Comprising NK Cell-Derived Exosomes and RSL3-Loaded Liposomes.ACS Appl Mater Interfaces. 2024 Jun 5;16(22):28193-28208. doi: 10.1021/acsami.4c04604. Epub 2024 May 22. ACS Appl Mater Interfaces. 2024. PMID: 38776411 Free PMC article.
-
Potential Role of Hsp70 and Activated NK Cells for Prediction of Prognosis in Glioblastoma Patients.Front Mol Biosci. 2021 May 17;8:669366. doi: 10.3389/fmolb.2021.669366. eCollection 2021. Front Mol Biosci. 2021. PMID: 34079819 Free PMC article.
-
Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC.Oncoimmunology. 2015 Aug 12;5(4):e1071008. doi: 10.1080/2162402X.2015.1071008. eCollection 2016 Apr. Oncoimmunology. 2015. PMID: 27141373 Free PMC article.
References
-
- Bauer S, Groh V, Wu J, Steinle A, Philips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. - PubMed
-
- Biassoni R, Cantoni C, and Falco M. et al. 1996 The human leukocyte antigen (HLA)-C-specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 183:645–650. - PMC - PubMed
-
- Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Penta J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–60. - PubMed
-
- Braud VM, Allan DS, and O'Callaghan CA. et al. 1998 HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 391:795–799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
