Origin of germ cells and formation of new primary follicles in adult human ovaries
- PMID: 15115550
- PMCID: PMC420494
- DOI: 10.1186/1477-7827-2-20
Origin of germ cells and formation of new primary follicles in adult human ovaries
Abstract
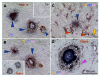
Recent reports indicate that functional mouse oocytes and sperm can be derived in vitro from somatic cell lines. We hypothesize that in adult human ovaries, mesenchymal cells in the tunica albuginea (TA) are bipotent progenitors with a commitment for both primitive granulosa and germ cells. We investigated ovaries of twelve adult women (mean age 32.8 +/- 4.1 SD, range 27-38 years) by single, double, and triple color immunohistochemistry. We show that cytokeratin (CK)+ mesenchymal cells in ovarian TA differentiate into surface epithelium (SE) cells by a mesenchymal-epithelial transition. Segments of SE directly associated with ovarian cortex are overgrown by TA, forming solid epithelial cords, which fragment into small (20 micron) epithelial nests descending into the lower ovarian cortex, before assembling with zona pellucida (ZP)+ oocytes. Germ cells can originate from SE cells which cover the TA. Small (10 micron) germ-like cells showing PS1 meiotically expressed oocyte carbohydrate protein are derived from SE cells via asymmetric division. They show nuclear MAPK immunoexpression, subsequently divide symmetrically, and enter adjacent cortical vessels. During vascular transport, the putative germ cells increase to oocyte size, and are picked-up by epithelial nests associated with the vessels. During follicle formation, extensions of granulosa cells enter the oocyte cytoplasm, forming a single paranuclear CK+ Balbiani body supplying all the mitochondria of the oocyte. In the ovarian medulla, occasional vessels show an accumulation of ZP+ oocytes (25-30 microns) or their remnants, suggesting that some oocytes degenerate. In contrast to males, adult human female gonads do not preserve germline type stem cells. This study expands our previous observations on the formation of germ cells in adult human ovaries. Differentiation of primitive granulosa and germ cells from the bipotent mesenchymal cell precursors of TA in adult human ovaries represents a most sophisticated adaptive mechanism created during the evolution of female reproduction. Our data indicate that the pool of primary follicles in adult human ovaries does not represent a static but a dynamic population of differentiating and regressing structures. An essential mission of such follicular turnover might be elimination of spontaneous or environmentally induced genetic alterations of oocytes in resting primary follicles.
Figures




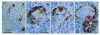










Comment in
-
Strong science challenges conventional wisdom: new perspectives on ovarian biology.Reprod Biol Endocrinol. 2004 Jun 8;2:28. doi: 10.1186/1477-7827-2-28. Reprod Biol Endocrinol. 2004. PMID: 15186496 Free PMC article. No abstract available.
References
-
- Waldeyer W. Eierstock und Ei Leipzig: Engelmann. 1870.
-
- Weissmann A. Die Continuitat des Keimplasmasals Grundlage einer Theorie der Vererbung. Jena: Fischer-Verlag; 1885.
-
- Franchi LL, Mandl AM, Zuckerman S. The development of the ovary and the process of oogenesis. In: Zuckerman S, editor. In The Ovary. London: Academic Press; 1962. pp. 1–88.
-
- Baker TG. Oogenesis and ovarian development. In: Balin H, Glasser S, editor. In Reproductive Biology. Amsterdam: Excerpta Medica; 1972. pp. 398–437.
-
- McLaren A. Signaling for germ cells. Genes Dev. 1999;13:373–376. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

