Immunohistochemical localization of integrin alpha V beta 3 and osteopontin suggests that they do not interact during embryo implantation in ruminants
- PMID: 15115551
- PMCID: PMC416490
- DOI: 10.1186/1477-7827-2-19
Immunohistochemical localization of integrin alpha V beta 3 and osteopontin suggests that they do not interact during embryo implantation in ruminants
Abstract
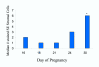
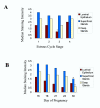
Background: It has been suggested that trophoblast attachment requires co-expression of integrin alpha V beta 3 and its ligand osteopontin at the fetal-maternal interface. Until now the expression patterns of integrin alpha V beta 3 and osteopontin in the pregnant bovine uterus were unknown. The objectives of this study were to localize integrin alpha V beta 3 and osteopontin in bovine and sheep endometrium during the periimplantation period and to compare the distribution patterns using antibodies that had not yet been tested in sheep.
Methods: Cell compartments within endometrial tissue sections were scored for immunohistochemical staining intensity and data were analyzed to determine the effects of day of pregnancy or cycle.
Results: In pregnant bovine endometrium, integrin alpha V beta 3 was detected in luminal epithelium, stroma, myometrium and smooth muscle. A strong band of immunoreactivity was observed in the subepithelial stroma of intercaruncular regions, but there was reduced reactivity in the caruncles and glands. Bovine trophoblast did not express integrin alpha V beta 3 at any stage of pregnancy. In ovine endometrium a different pattern of staining for integrin alpha V beta 3 was observed. Reactivity was not present in the luminal epithelium or trophoblast. There was strong staining of the deep glands and no reactivity in the superficial glands. Osteopontin distribution was similar for sheep and cattle. For both species, apical staining was present on the luminal epithelium and glands and on embryonic tissues.
Conclusion: In ruminants, integrin alpha V beta 3 and osteopontin do not co-localize at the fetal-maternal interface indicating that these proteins could not interact to facilitate embryo attachment as has been proposed in other species.
Figures




Similar articles
-
Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep.Biol Reprod. 2001 Sep;65(3):820-8. doi: 10.1095/biolreprod65.3.820. Biol Reprod. 2001. PMID: 11514347
-
αv β3 Integrin may participate in conceptus attachment by regulating morphologic changes in the endometrium during peri-implantation in ovine.Reprod Domest Anim. 2011 Oct;46(5):840-7. doi: 10.1111/j.1439-0531.2011.01752.x. Epub 2011 May 24. Reprod Domest Anim. 2011. PMID: 21605197
-
Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration.Biol Reprod. 2009 Nov;81(5):814-25. doi: 10.1095/biolreprod.109.078600. Epub 2009 Jul 1. Biol Reprod. 2009. PMID: 19571258
-
Adhesion molecules and implantation.J Reprod Immunol. 2002 May-Jun;55(1-2):101-12. doi: 10.1016/s0165-0378(01)00139-5. J Reprod Immunol. 2002. PMID: 12062825 Review.
-
Understanding placentation in ruminants: a review focusing on cows and sheep.Reprod Fertil Dev. 2023 Dec;36(2):93-111. doi: 10.1071/RD23119. Reprod Fertil Dev. 2023. PMID: 38064193 Review.
Cited by
-
The Early Stages of Implantation and Placentation in the Pig.Adv Anat Embryol Cell Biol. 2021;234:61-89. doi: 10.1007/978-3-030-77360-1_5. Adv Anat Embryol Cell Biol. 2021. PMID: 34694478
-
Cytokines, growth factors and macromolecules as mediators of implantation in mammalian species.Int J Vet Sci Med. 2017 Dec 19;6(Suppl):S6-S14. doi: 10.1016/j.ijvsm.2017.12.001. eCollection 2018. Int J Vet Sci Med. 2017. PMID: 30761315 Free PMC article. Review.
References
-
- Amoroso EC. Placentation. In: Parkes AS, editor. In Marshall's Physiology of Reproduction. London: Longmans Green; 1952. pp. 127–309.
-
- King GJ, Atkinson BA, Robertson HA. Implantation and early placentation in domestic ungulates. J Reprod Fertil Suppl. 1982;31:17–30. - PubMed
-
- Atkinson BA, King GJ, Amoroso EC. Development of the caruncular and intercaruncular regions in the bovine endometrium. Biol Reprod. 1984;30:763–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

