Involvement of monkey inferior colliculus in spatial hearing
- PMID: 15115809
- PMCID: PMC6729282
- DOI: 10.1523/JNEUROSCI.0199-04.2004
Involvement of monkey inferior colliculus in spatial hearing
Abstract
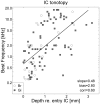
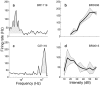
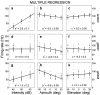
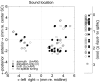
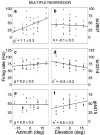
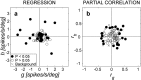
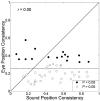
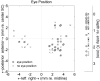
The midbrain inferior colliculus (IC) is implicated in coding sound location, but evidence from behaving primates is scarce. Here we report single-unit responses to broadband sounds that were systematically varied within the two-dimensional (2D) frontal hemifield, as well as in sound level, while monkeys fixated a central visual target. Results show that IC neurons are broadly tuned to both sound-source azimuth and level in a way that can be approximated by multiplicative, planar modulation of the firing rate of the cell. In addition, a fraction of neurons also responded to elevation. This tuning, however, was more varied: some neurons were sensitive to a specific elevation; others responded to elevation in a monotonic way. Multiple-linear regression parameters varied from cell to cell, but the only topography encountered was a dorsoventral tonotopy. In a second experiment, we presented sounds from straight ahead while monkeys fixated visual targets at different positions. We found that auditory responses in a fraction of IC cells were weakly, but systematically, modulated by 2D eye position. This modulation was absent in the spontaneous firing rates, again suggesting a multiplicative interaction of acoustic and eye-position inputs. Tuning parameters to sound frequency, location, intensity, and eye position were uncorrelated. On the basis of simulations with a simple neural network model, we suggest that the population of IC cells could encode the head-centered 2D sound location and enable a direct transformation of this signal into the eye-centered topographic motor map of the superior colliculus. Both signals are required to generate rapid eye-head orienting movements toward sounds.
Figures




Similar articles
-
Eye position influences auditory responses in primate inferior colliculus.Neuron. 2001 Feb;29(2):509-18. doi: 10.1016/s0896-6273(01)00222-7. Neuron. 2001. PMID: 11239439
-
Representation of eye position in primate inferior colliculus.J Neurophysiol. 2006 Mar;95(3):1826-42. doi: 10.1152/jn.00857.2005. Epub 2005 Oct 12. J Neurophysiol. 2006. PMID: 16221747
-
Influence of head position on the spatial representation of acoustic targets.J Neurophysiol. 1999 Jun;81(6):2720-36. doi: 10.1152/jn.1999.81.6.2720. J Neurophysiol. 1999. PMID: 10368392
-
[Perception and selectivity of sound duration in the central auditory midbrain].Sheng Li Xue Bao. 2010 Aug 25;62(4):309-16. Sheng Li Xue Bao. 2010. PMID: 20717631 Review. Chinese.
-
Multisensory guidance of orienting behavior.Hear Res. 2009 Dec;258(1-2):106-12. doi: 10.1016/j.heares.2009.05.008. Epub 2009 Jun 9. Hear Res. 2009. PMID: 19520151 Free PMC article. Review.
Cited by
-
Influence of static eye and head position on tone-evoked gaze shifts.J Neurosci. 2011 Nov 30;31(48):17496-504. doi: 10.1523/JNEUROSCI.5030-10.2011. J Neurosci. 2011. PMID: 22131411 Free PMC article.
-
Segregating two simultaneous sounds in elevation using temporal envelope: Human psychophysics and a physiological model.J Acoust Soc Am. 2015 Jul;138(1):33-43. doi: 10.1121/1.4922224. J Acoust Soc Am. 2015. PMID: 26233004 Free PMC article.
-
Auditory spatial perception dynamically realigns with changing eye position.J Neurosci. 2007 Sep 19;27(38):10249-58. doi: 10.1523/JNEUROSCI.0938-07.2007. J Neurosci. 2007. PMID: 17881531 Free PMC article.
-
Comparison of gain-like properties of eye position signals in inferior colliculus versus auditory cortex of primates.Front Integr Neurosci. 2010 Aug 20;4:121. doi: 10.3389/fnint.2010.00121. eCollection 2010. Front Integr Neurosci. 2010. PMID: 20838470 Free PMC article.
-
Alignment of sound localization cues in the nucleus of the brachium of the inferior colliculus.J Neurophysiol. 2014 Jun 15;111(12):2624-33. doi: 10.1152/jn.00885.2013. Epub 2014 Mar 26. J Neurophysiol. 2014. PMID: 24671535 Free PMC article.
References
-
- Aitkin L, Martin R (1990) Neurons in the inferior colliculus of cats sensitive to sound-source elevation. Hear Res 50: 97-105. - PubMed
-
- Andersen RA, Roth GL, Aitkin LM, Merzenich MM (1980) The efferent projections of the central nucleus and the pericentral nucleus of the inferior colliculus in the cat. J Comp Neurol 194: 649-662. - PubMed
-
- Andre-Deshays C, Berthoz A, Revel M (1988) Eye-head coupling in humans. I. Simultaneous recording of isolated motor units in dorsal neck muscles and horizontal eye movements. Exp Brain Res 69: 399-406. - PubMed
-
- Binns KE, Grant S, Withington DJ, Keating MJ (1992) A topographic representation of auditory space in the external nucleus of the inferior colliculus of the guinea-pig. Brain Res 589: 231-242. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
