Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor
- PMID: 15115821
- PMCID: PMC6729276
- DOI: 10.1523/JNEUROSCI.3920-03.2004
Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor
Abstract
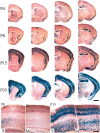
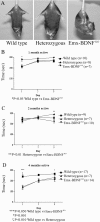
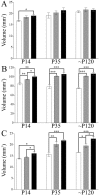
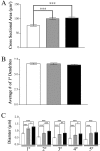
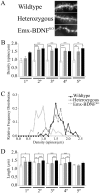
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, modulates neuronal survival, differentiation, and synaptic function. Reduced BDNF expression in the cortex caused by mutation of the huntingtin gene has been suggested to play a role in the striatal degeneration observed in Huntington's disease. BDNF expression rises dramatically in the cortex during the first few weeks of postnatal life in mice. Previously, it has been impossible to study the specific long-term effects of BDNF absence on CNS structures because of the early postnatal lethality of BDNF-/- mice. Mice harboring a floxed BDNF gene were bred with Emx1(IREScre/+) mice to generate Emx-BDNF(KO) mice that lack cortical BDNF but are viable. Adult Emx-BDNF(KO) mice display a hindlimb clasping phenotype similar to that observed in mouse models of Huntington's disease. The striatum of postnatal Emx-BDNF(KO) mice was reduced in volume compared with controls, and the most abundant neuron type of the striatum, medium spiny neurons (MSNs), had shrunken cell somas, thinner dendrites, and fewer dendritic spines at 35 d of age. Although significant striatal neuron losses were not detected at 35 or 120 d postnatal, 35% of striatal neurons were missing in Emx-BDNF(KO) mice aged beyond 1 year. Thus, cortical BDNF, although not required for the generation or near-term survival of MSN, is necessary for normal striatal neuron dendrite morphology during the period when BDNF expression rises in the cortex. Furthermore, a long-term in vivo requirement for cortical BDNF in supporting the survival of MSNs is revealed.
Figures


References
-
- Alberch J, Perez-Navarro E, Canals JM (2002) Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull 57: 817-822. - PubMed
-
- Altar CA, DiStefano PS (1998) Neurotrophin trafficking by anterograde transport. Trends Neurosci 21: 433-437. - PubMed
-
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389: 856-860. - PubMed
-
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, et al. (1993) The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet 4: 398-403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
