Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent
- PMID: 15116067
- PMCID: PMC424358
- DOI: 10.1038/sj.emboj.7600159
Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent
Abstract
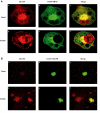
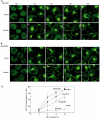
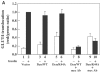
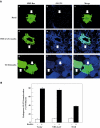
Following biosynthesis, both GLUT1 and VSV-G proteins appear rapidly (2-3 h) at the plasma membrane, whereas GLUT4 is retained in intracellular membrane compartments and does not display any significant insulin responsiveness until 6-9 h. Surprisingly, the acquisition of insulin responsiveness did not require plasma membrane endocytosis, as expression of a dominant-interfering dynamin mutant (Dyn/K44A) had no effect on the insulin-stimulated GLUT4 translocation. Furthermore, expression of endocytosis-defective GLUT4 mutants or continuous surface labeling with an exofacial specific antibody demonstrated that GLUT4 did not transit the cell surface prior to the acquisition of insulin responsiveness. The expression of a dominant-interfering GGA mutant (VHS-GAT) had no effect on the trafficking of newly synthesized GLUT1 or VSV-G protein to the plasma membrane, but completely blocked the insulin-stimulated translocation of newly synthesized GLUT4. Furthermore, in vitro budding of GLUT4 vesicles but not GLUT1 or the transferrin receptor was inhibited by VHS-GAT. Together, these data demonstrate that following biosynthesis, GLUT4 directly sorts and traffics to the insulin-responsive storage compartment through a specific GGA-sensitive process.
Figures





References
-
- Al-Hasani H, Hinck CS, Cushman SW (1998) Endocytosis of the glucose transporter GLUT4 is mediated by the GTPase dynamin. J Biol Chem 273: 17504–17510 - PubMed
-
- Baumann CA, Ribon V, Kanzaki K, Thurmond DC, Mora S, Shigematsu S, Bickel PE, Pessin JE, Saltiel AR (2000) CAP defines a second signaling pathway required for insulin-stimulated glucose transport. Nature 407: 202–207 - PubMed
-
- Bergmann JE (1989) Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol 32: 85–110 - PubMed
-
- Bryant NJ, Govers R, James DE (2002) Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3: 267–277 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

