Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype
- PMID: 15118091
- PMCID: PMC406472
- DOI: 10.1073/pnas.0402133101
Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype
Abstract
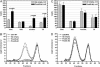
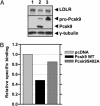
Proprotein convertase subtilisin kexin 9 (Pcsk9) is a subtilisin serine protease with a putative role in cholesterol metabolism. Pcsk9 expression is down-regulated by dietary cholesterol, and mutations in Pcsk9 have been associated with a form of autosomal dominant hypercholesterolemia. To study the function of Pcsk9 in mice, an adenovirus constitutively expressing murine Pcsk9 (Pcsk9-Ad) was used. Pcsk9 overexpression in wild-type mice caused a 2-fold increase in plasma total cholesterol and a 5-fold increase in non-high-density lipoprotein (HDL) cholesterol, with no increase in HDL cholesterol, as compared with mice infected with a control adenovirus. Fast protein liquid chromatography analysis showed that the increase in non-HDL cholesterol was due to an increase in low-density lipoprotein (LDL) cholesterol. This effect appeared to depend on the LDL receptor (LDLR) because LDLR knockout mice infected with Pcsk9-Ad had no change in plasma cholesterol levels as compared with knockout mice infected with a control adenovirus. Furthermore, whereas overexpression of Pcsk9 had no effect on LDLR mRNA levels, there was a near absence of LDLR protein in animals overexpressing Pcsk9. These results were confirmed in vitro by the demonstration that transfection of Pcsk9 in McA-RH7777 cells caused a reduction in LDLR protein and LDL binding. In summary, these results indicate that overexpression of Pcsk9 interferes with LDLR-mediated LDL cholesterol uptake. Because Pcsk9 and LDLR are coordinately regulated by cholesterol, Pcsk9 may be involved in a novel mechanism to modulate LDLR function by an alternative pathway than classic cholesterol inhibition of sterol regulatory element binding protein-mediated transcription.
Figures


References
-
- Hopkins, P. N. (1992) Am. J. Clin. Nutr. 55, 1060-1070. - PubMed
-
- Herron, K. L., Vega-Lopez, S., Conde, K., Ramjiganesh, T., Shachter, N. S. & Fernandez, M. L. (2003) J. Nutr. 133, 1036-1042. - PubMed
-
- Maxwell, K. N., Soccio, R. E., Duncan, E. M., Sehayek, E. & Breslow, J. L. (2003) J. Lipid Res. 44, 2109-2119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

