K+ channels as targets for specific immunomodulation
- PMID: 15120495
- PMCID: PMC2749963
- DOI: 10.1016/j.tips.2004.03.010
K+ channels as targets for specific immunomodulation
Abstract
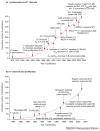
The voltage-gated Kv1.3 channel and the Ca(2+)-activated IKCa1 K(+) channel are expressed in T cells in a distinct pattern that depends on the state of lymphocyte activation and differentiation. The channel phenotype changes during the progression from the resting to the activated cell state and from naïve to effector memory cells, affording promise for specific immunomodulatory actions of K(+) channel blockers. In this article, we review the functional roles of these channels in both naïve cells and memory cells, describe the development of selective inhibitors of Kv1.3 and IKCa1 channels, and provide a rationale for the potential therapeutic use of these inhibitors in immunological disorders.
Figures



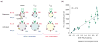
References
-
- Iversen JG. Unidirectional K+ fluxes in rat thymocytes stimulated by concanavalin A. J Cell Physiol. 1976;89:267–276. - PubMed
-
- DeCoursey TE, et al. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. - PubMed
-
- Matteson DR, Deutsch C. K+ channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984;307:468–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

