A role for SNAP-25 but not VAMPs in store-mediated Ca2+ entry in human platelets
- PMID: 15121806
- PMCID: PMC1664928
- DOI: 10.1113/jphysiol.2004.064899
A role for SNAP-25 but not VAMPs in store-mediated Ca2+ entry in human platelets
Abstract
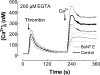
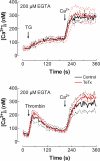
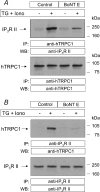
Store-mediated Ca2+ entry (SMCE) is a major mechanism for Ca2+ influx in non-excitable cells. Recently, a conformational coupling mechanism allowing coupling between transient receptor potential channels (TRPCs) and IP3 receptors has been proposed to activate SMCE. Here we have investigated the role of two soluble N-ethylmaleimide-sensitive-factor attachment protein receptors (SNAREs), which are involved in membrane trafficking and docking, in SMCE in human platelets. We found that the synaptosome-associated protein (SNAP-25) and the vesicle-associated membrane proteins (VAMP) coimmunoprecipitate with hTRPC1 in platelets. Treatment with botulinum toxin (BoNT) E or with tetanus toxin (TeTx), induced cleavage and inactivation of SNAP-25 and VAMPs, respectively. BoNTs significantly reduced thapsigargin- (TG) and agonist-evoked SMCE. Treatment with BoNTs once SMCE had been activated decreased Ca2+ entry, indicating that SNAP-25 is required for the activation and maintenance of SMCE. In contrast, treatment with TeTx had no effect on either the activation or the maintenance of SMCE in platelets. Finally, treatment with BoNT E impaired the coupling between naturally expressed hTRPC1 and IP3 receptor type II in platelets. From these findings we suggest SNAP-25 has a role in SMCE in human platelets.
Figures




Similar articles
-
Rapid agonist-evoked coupling of type II Ins(1,4,5)P3 receptor with human transient receptor potential (hTRPC1) channels in human platelets.Biochem J. 2003 Nov 1;375(Pt 3):697-704. doi: 10.1042/BJ20030929. Biochem J. 2003. PMID: 12908873 Free PMC article.
-
Cleavage of SNAP-25 and VAMP-2 impairs store-operated Ca2+ entry in mouse pancreatic acinar cells.Am J Physiol Cell Physiol. 2005 Jan;288(1):C214-21. doi: 10.1152/ajpcell.00241.2004. Epub 2004 Sep 8. Am J Physiol Cell Physiol. 2005. PMID: 15355848
-
The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks Ca2+ entry channels in human platelets: cautions for its use in studying Ca2+ influx.Cell Calcium. 2001 Nov;30(5):323-9. doi: 10.1054/ceca.2001.0239. Cell Calcium. 2001. PMID: 11733938
-
A role for the actin cytoskeleton in the initiation and maintenance of store-mediated calcium entry in human platelets.Trends Cardiovasc Med. 2000 Nov;10(8):327-32. doi: 10.1016/s1050-1738(01)00073-1. Trends Cardiovasc Med. 2000. PMID: 11369258 Review.
-
Store-operated Ca2+ entry: vesicle fusion or reversible trafficking and de novo conformational coupling?J Cell Physiol. 2005 Nov;205(2):262-9. doi: 10.1002/jcp.20399. J Cell Physiol. 2005. PMID: 15880447 Review.
Cited by
-
Localization of TRPC1 channel in the sinus endothelial cells of rat spleen.Histochem Cell Biol. 2005 Jun;123(4-5):347-56. doi: 10.1007/s00418-004-0741-6. Epub 2005 Apr 27. Histochem Cell Biol. 2005. PMID: 15856275
-
Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells.Toxins (Basel). 2010 Apr;2(4):683-737. doi: 10.3390/toxins2040683. Epub 2010 Apr 15. Toxins (Basel). 2010. PMID: 22069606 Free PMC article. Review.
-
Advances in Intracellular Calcium Signaling Reveal Untapped Targets for Cancer Therapy.Biomedicines. 2021 Aug 24;9(9):1077. doi: 10.3390/biomedicines9091077. Biomedicines. 2021. PMID: 34572262 Free PMC article. Review.
-
PAR-1-stimulated factor IXa binding to a small platelet subpopulation requires a pronounced and sustained increase of cytoplasmic calcium.Biochemistry. 2006 Jun 13;45(23):7289-98. doi: 10.1021/bi060294m. Biochemistry. 2006. PMID: 16752917 Free PMC article.
-
The reverse roles of transient receptor potential canonical channel-3 and -6 in neuronal death following pilocarpine-induced status epilepticus.Cell Mol Neurobiol. 2013 Jan;33(1):99-109. doi: 10.1007/s10571-012-9875-6. Epub 2012 Aug 28. Cell Mol Neurobiol. 2013. PMID: 22926417 Free PMC article.
References
-
- Bernstein AM, Whiteheart SW. Identification of a cellubrevin/vesicle associated membrane protein 3 homologue in human platelets. Blood. 1999;93:571–579. - PubMed
-
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

