Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes
- PMID: 15121854
- PMCID: PMC400463
- DOI: 10.1128/MCB.24.10.4351-4360.2004
Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes
Abstract
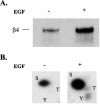
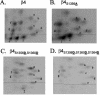
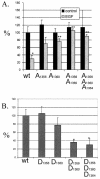
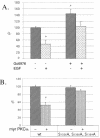
Although the regulation of hemidesmosome dynamics during processes such as epithelial migration, wound healing, and carcinoma invasion is important, the mechanisms involved are poorly understood. The integrin alpha 6 beta 4 is an essential component of the hemidesmosome and a target of such regulation. Epidermal growth factor (EGF) can induce hemidesmosome disassembly by a mechanism that involves serine phosphorylation of the beta 4 integrin subunit. Using a combination of biochemical and mutational analyses, we demonstrate that EGF induces the phosphorylation of three specific serine residues (S(1356), S(1360), and S(1364)) located within the connecting segment of the beta 4 subunit and that phosphorylation on these residues accounts for the bulk of beta 4 phosphorylation stimulated by EGF. Importantly, phosphorylation of these serines is critical for the ability of EGF to disrupt hemidesmosomes. Using COS-7 cells, which assemble hemidesmosomes type II upon exogenous expression of the alpha 6 beta 4 integrin, we observed that expression of a beta 4 construct containing Ser-->Ala mutations of S(1356), S(1360), and S(1364) reduced the ability of EGF to disrupt hemidesmosomes and that this effect appears to involve cooperation among these phosphorylation sites. Moreover, expression of Ser-->Asp mutants that mimic constitutive phosphorylation reduced hemidesmosome formation. Protein kinase C-alpha (PKC-alpha) is the kinase responsible for phosphorylating at least two of these serines, based on in vitro kinase assays, peptide mapping, and mutational analysis. Together, these results highlight the importance of serine phosphorylation in regulating type II hemidesmosome disassembly, implicate a cluster of serine residues within the connecting segment of beta 4, and argue for a key role for PKC-alpha in regulating these structures.
Figures




References
-
- Alt, A., M. Ohba, L. Li, M. Gartsbein, A. Belanger, M. F. Denning, T. Kuroki, S. H. Yuspa, and T. Tennenbaum. 2001. Protein kinase Cdelta-mediated phosphorylation of alpha6beta4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res. 61:4591-4598. - PubMed
-
- Beaulieu, J. F. 1997. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 31:1-78. - PubMed
-
- Borradori, L., P. J. Koch, C. M. Niessen, S. Erkeland, M. R. van Leusden, and A. Sonnenberg. 1997. The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the beta4 integrin subunit. J. Cell Biol. 136:1333-1347. - PMC - PubMed
-
- Borradori, L., and A. Sonnenberg. 1999. Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Investig. Dermatol. 112:411-418. - PubMed
-
- Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
