The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification
- PMID: 15121860
- PMCID: PMC400456
- DOI: 10.1128/MCB.24.10.4417-4427.2004
The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification
Abstract
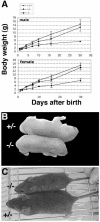
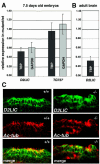
There are five members of the RFX family of transcription factors in mammals. While RFX5 plays a well-defined role in the immune system, the functions of RFX1 to RFX4 remain largely unknown. We have generated mice with a deletion of the Rfx3 gene. RFX3-deficient mice exhibit frequent left-right (LR) asymmetry defects leading to a high rate of embryonic lethality and situs inversus in surviving adults. In vertebrates, specification of the LR body axis is controlled by monocilia in the embryonic node, and defects in nodal cilia consequently result in abnormal LR patterning. Consistent with this, Rfx3 is expressed in ciliated cells of the node and RFX3-deficient mice exhibit a pronounced defect in nodal cilia. In contrast to the case for wild-type embryos, for which we document for the first time a twofold increase in the length of nodal cilia during development, the cilia are present but remain markedly stunted in mutant embryos. Finally, we show that RFX3 regulates the expression of D2lic, the mouse orthologue of a Caenorhabditis elegans gene that is implicated in intraflagellar transport, a process required for the assembly and maintenance of cilia. In conclusion, RFX3 is essential for the differentiation of nodal monocilia and hence for LR body axis determination.
Figures



References
-
- Bartoloni, L., J. L. Blouin, Y. Pan, C. Gehrig, A. K. Maiti, N. Scamuffa, C. Rossier, M. Jorissen, M. Armengot, M. Meeks, H. M. Mitchison, E. M. Chung, C. D. Delozier-Blanchet, W. J. Craigen, and S. E. Antonarakis. 2002. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA 99:10282-10286. - PMC - PubMed
-
- Blackshear, P. J., J. P. Graves, D. J. Stumpo, I. Cobos, J. L. Rubenstein, and D. C. Zeldin. 2003. Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development 130:4539-4552. - PubMed
-
- Brody, S. L., X. H. Yan, M. K. Wuerffel, S. K. Song, and S. D. Shapiro. 2000. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell. Mol. Biol. 23:45-51. - PubMed
-
- Brueckner, M. 2001. Cilia propel the embryo in the right direction. Am. J. Med. Genet. 101:339-344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
