Calcium interacts with antifreeze proteins and chitinase from cold-acclimated winter rye
- PMID: 15122015
- PMCID: PMC429390
- DOI: 10.1104/pp.103.038158
Calcium interacts with antifreeze proteins and chitinase from cold-acclimated winter rye
Abstract
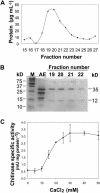
During cold acclimation, winter rye (Secale cereale) plants accumulate pathogenesis-related proteins that are also antifreeze proteins (AFPs) because they adsorb onto ice and inhibit its growth. Although they promote winter survival in planta, these dual-function AFPs proteins lose activity when stored at subzero temperatures in vitro, so we examined their stability in solutions containing CaCl2, MgCl2, or NaCl. Antifreeze activity was unaffected by salts before freezing, but decreased after freezing and thawing in CaCl2 and was recovered by adding a chelator. Ca2+ enhanced chitinase activity 3- to 5-fold in unfrozen samples, although hydrolytic activity also decreased after freezing and thawing in CaCl2. Native PAGE, circular dichroism, and Trp fluorescence experiments showed that the AFPs partially unfold after freezing and thawing, but they fold more compactly or aggregate in CaCl2. Ruthenium red, which binds to Ca(2+)-binding sites, readily stained AFPs in the absence of Ca2+, but less stain was visible after freezing and thawing AFPs in CaCl2. We conclude that the structure of AFPs changes during freezing and thawing, creating new Ca(2+)-binding sites. Once Ca2+ binds to those sites, antifreeze activity, chitinase activity and ruthenium red binding are all inhibited. Because free Ca2+ concentrations are typically low in the apoplast, antifreeze activity is probably stable to freezing and thawing in planta. Ca2+ may regulate chitinase activity if concentrations are increased locally by release from pectin or interaction with Ca(2+)-binding proteins. Furthermore, antifreeze activity can be easily maintained in vitro by including a chelator during frozen storage.
Figures






Similar articles
-
Antifreeze proteins enable plants to survive in freezing conditions.J Biosci. 2014 Dec;39(5):931-44. doi: 10.1007/s12038-014-9468-2. J Biosci. 2014. PMID: 25431421 Review.
-
Antifreeze proteins modify the freezing process in planta.Plant Physiol. 2005 May;138(1):330-40. doi: 10.1104/pp.104.058628. Epub 2005 Apr 1. Plant Physiol. 2005. PMID: 15805474 Free PMC article.
-
Chitinase genes responsive to cold encode antifreeze proteins in winter cereals.Plant Physiol. 2000 Nov;124(3):1251-64. doi: 10.1104/pp.124.3.1251. Plant Physiol. 2000. PMID: 11080301 Free PMC article.
-
Immunolocalization of Antifreeze Proteins in Winter Rye Leaves, Crowns, and Roots by Tissue Printing.Plant Physiol. 1996 Mar;110(3):845-857. doi: 10.1104/pp.110.3.845. Plant Physiol. 1996. PMID: 12226223 Free PMC article.
-
Antifreeze proteins in overwintering plants: a tale of two activities.Trends Plant Sci. 2004 Aug;9(8):399-405. doi: 10.1016/j.tplants.2004.06.007. Trends Plant Sci. 2004. PMID: 15358271 Review.
Cited by
-
Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium.Plant Physiol. 2009 May;150(1):229-43. doi: 10.1104/pp.108.131524. Epub 2009 Mar 11. Plant Physiol. 2009. PMID: 19279198 Free PMC article.
-
Refolding of β-stranded class I chitinases of Hippophae rhamnoides enhances the antifreeze activity during cold acclimation.PLoS One. 2014 Mar 13;9(3):e91723. doi: 10.1371/journal.pone.0091723. eCollection 2014. PLoS One. 2014. PMID: 24626216 Free PMC article.
-
Antifreeze proteins enable plants to survive in freezing conditions.J Biosci. 2014 Dec;39(5):931-44. doi: 10.1007/s12038-014-9468-2. J Biosci. 2014. PMID: 25431421 Review.
-
Transcriptomic and Metabolomic Analyses Reveal a Potential Mechanism to Improve Soybean Resistance to Anthracnose.Front Plant Sci. 2022 Apr 27;13:850829. doi: 10.3389/fpls.2022.850829. eCollection 2022. Front Plant Sci. 2022. PMID: 35574068 Free PMC article.
-
Characterization of cold-responsive extracellular chitinase in bromegrass cell cultures and its relationship to antifreeze activity.Plant Physiol. 2008 May;147(1):391-401. doi: 10.1104/pp.106.081497. Epub 2008 Mar 21. Plant Physiol. 2008. PMID: 18359848 Free PMC article.
References
-
- Achenbach JC, Ewart KV (2002) Structural and functional characterization of a C-type lectin-like antifreeze protein from rainbow smelt (Osmerus mordax). Eur J Biochem 269: 1219–1226 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 341–374 - PubMed
-
- Bush DS (1995) Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol 46: 95–122
-
- Campbell ID, Dwek RA (1984) Biological Spectroscopy. Benjamin/Cummings Publishing, Menlo Park, CA, pp 255–277
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

