Extensive phenotypic variation in early flowering mutants of Arabidopsis
- PMID: 15122022
- PMCID: PMC429349
- DOI: 10.1104/pp.104.039453
Extensive phenotypic variation in early flowering mutants of Arabidopsis
Abstract
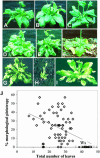
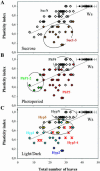
Flowering time, the major regulatory transition of plant sequential development, is modulated by multiple endogenous and environmental factors. By phenotypic profiling of 80 early flowering mutants of Arabidopsis, we examine how mutational reduction of floral repression is associated with changes in phenotypic plasticity and stability. Flowering time measurements in mutants reveal deviations from the linear relationship between the number of leaves and number of days to bolting described for natural accessions and late flowering mutants. The deviations correspond to relative early bolting and relative late bolting phenotypes. Only a minority of mutants presents no detectable phenotypic variation. Mutants are characterized by a broad release of morphological pleiotropy under short days, with leaf characters being most variable. They also exhibit changes in phenotypic plasticity across environments for florigenic-related responses, including the reaction to light and dark, photoperiodic behavior, and Suc sensitivity. Morphological pleiotropy and plasticity modifications are differentially distributed among mutants, resulting in a large diversity of multiple phenotypic changes. The pleiotropic effects observed may indicate that floral repression defects are linked to global developmental perturbations. This first, to our knowledge, extensive characterization of phenotypic variation in early flowering mutants correlates with the reports that most factors recruited in floral repression at the molecular genetic level correspond to ubiquitous regulators. We discuss the importance of functional ubiquity for floral repression with respect to robustness and flexibility of network biological systems.
Figures




References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Amzallag GN (2001) Data analysis in plant physiology: are we missing the reality? Plant Cell Env 24: 881–890
-
- Armbruster WS, Schwaergerle KE (1996) Causes of covariation of phenotypic traits among populations. J Evol Biol 9: 261–276
-
- Bagnall DJ (1993) Light quality and vernalization interact in controlling late flowering in Arabidopsis ecotypes and mutants. Ann Bot (Lond) 71: 75–83
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

