Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants
- PMID: 15122040
- PMCID: PMC429367
- DOI: 10.1104/pp.103.036988
Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants
Abstract
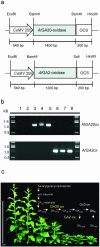
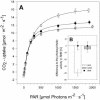
Gibberellins (GAs) are involved in regulation of many aspects during plant development. To investigate the impact of altered GA levels on plant growth and metabolism, transgenic tobacco (Nicotiana tabacum) plants have been engineered to express either a GA20-oxidase (AtGA20-ox) or a GA2-oxidase (AtGA2-ox) gene from Arabidopsis under control of the cauliflower mosaic virus 35S promoter. Resulting plants were characterized by elongated or stunted shoot growth, respectively, indicating changes in the content of bioactive GAs. In accordance with the effect on plant growth, biomass production was increased or decreased in AtGA20-ox or AtGA2-ox plants, respectively, and was found to be positively correlated with the rate of photosynthesis as determined at the whole plant level. Differences in dry matter accumulation were most likely due to changes in lignin deposition as indicated by histochemical staining and quantitative measurements. Altered lignification of transgenic plants was paralleled by up- or down-regulation of the expression of lignin biosynthetic genes. Short-term GA3 feeding of excised petioles induced lignin formation in the absence of a transcriptional activation of pathway-specific genes. Thus, short-term GA treatment mediates lignin deposition most likely by polymerization of preformed monomers, whereas long-term effects on lignification involve elevated production of precursors by transcriptional stimulation of the biosynthetic pathway. Interestingly, analysis of stem cross sections revealed a differential effect of GA on the formation of xylem and pith cells. The number of lignified vessels was increased in AtGA20-ox plants pointing to a stimulation of xylem formation while the number of pith cells declined indicating a negative regulation.
Figures




References
-
- Ashraf M, Karim F, Rasul E (2002) Interactive effects of gibberellic acid (GA3) and salt stress on growth, ion accumulation and photosynthetic capacity of two spring wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Plant Growth Regul 36: 49–59
-
- Campbell MM, Ellis BE (1992) Fungal elicitor mediated responses in pine cell cultures I. Induction of phenylpropanoid metabolism. Planta 186: 409–417 - PubMed
-
- Carrera E, Bou J, Garcia-Martinez JL, Prat S (2000) Changes in GA20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J 22: 247–256 - PubMed
-
- Coles JP, Phillips AL, Croker SJ, Garcia-Lepe R, Lewis MJ, Hedden P (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase. Plant J 17: 547–556 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

