Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat
- PMID: 15123645
- PMCID: PMC1828032
- DOI: 10.1074/jbc.M404167200
Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat
Abstract
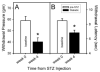
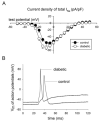
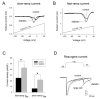
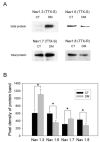
Diabetic neuropathy is a common form of peripheral neuropathy, yet the mechanisms responsible for pain in this disease are poorly understood. Alterations in the expression and function of voltage-gated tetrodotoxin-resistant (TTX-R) sodium channels have been implicated in animal models of neuropathic pain, including models of diabetic neuropathy. We investigated the expression and function of TTX-sensitive (TTX-S) and TTX-R sodium channels in dorsal root ganglion (DRG) neurons and the responses to thermal hyperalgesia and mechanical allodynia in streptozotocin-treated rats between 4-8 weeks after onset of diabetes. Diabetic rats demonstrated a significant reduction in the threshold for escape from innocuous mechanical pressure (allodynia) and a reduction in the latency to withdrawal from a noxious thermal stimulus (hyperalgesia). Both TTX-S and TTX-R sodium currents increased significantly in small DRG neurons isolated from diabetic rats. The voltage-dependent activation and steady-state inactivation curves for these currents were shifted negatively. TTX-S currents induced by fast or slow voltage ramps increased markedly in neurons from diabetic rats. Immunoblots and immunofluorescence staining demonstrated significant increases in the expression of Na(v)1.3 (TTX-S) and Na(v) 1.7 (TTX-S) and decreases in the expression of Na(v) 1.6 (TTX-S) and Na(v)1.8 (TTX-R) in diabetic rats. The level of serine/threonine phosphorylation of Na(v) 1.6 and In Na(v)1.8 increased in response to diabetes. addition, increased tyrosine phosphorylation of Na(v)1.6 and Na(v)1.7 was observed in DRGs from diabetic rats. These results suggest that both TTX-S and TTX-R sodium channels play important roles and that differential phosphorylation of sodium channels involving both serine/threonine and tyrosine sites contributes to painful diabetic neuropathy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

