Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes
- PMID: 15123742
- PMCID: PMC2211911
- DOI: 10.1084/jem.20040274
Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes
Abstract
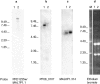
Parasite-encoded variant surface antigens (VSAs) like the var gene-encoded Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family are responsible for antigenic variation and infected red blood cell (RBC) cytoadhesion in P. falciparum malaria. Parasites causing severe malaria in nonimmune patients tend to express a restricted subset of VSA (VSA(SM)) that differs from VSA associated with uncomplicated malaria and asymptomatic infection (VSA(UM)). We compared var gene transcription in unselected P. falciparum clone 3D7 expressing VSA(UM) to in vitro-selected sublines expressing VSA(SM) to identify PfEMP1 responsible for the VSA(SM) phenotype. Expression of VSA(SM) was accompanied by up-regulation of Group A var genes. The most prominently up-regulated Group A gene (PFD1235w/MAL7P1.1) was translated into a protein expressed on the infected RBC surface. The proteins encoded by Group A var genes, such as PFD1235w/MAL7P1.1, appear to be involved in the pathogenesis of severe disease and are thus attractive candidates for a vaccine against life-threatening P. falciparum malaria.
Figures




References
-
- Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673–707. - PubMed
-
- Bull, P.C., M. Kortok, O. Kai, F. Ndungu, A. Ross, B.S. Lowe, C.I. Newbold, and K. Marsh. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252–259. - PubMed
-
- Nielsen, M.A., T. Staalsoe, J.A.L. Kurtzhals, B.Q. Goka, D. Dodoo, M. Alifrangis, T.G. Theander, B.D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and non-severe malaria and is modified by acquired immunity. J. Immunol. 168:3444–3450. - PubMed
-
- Nielsen, M.A., L.S. Vestergaard, J. Lusingu, J.A.L. Kurtzhals, H. Giha, B. Grevstad, B.Q. Goka, M.M. Lemnge, J.B. Jensen, B.D. Akanmori, et al. 2004. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect. Immun. In press. - PMC - PubMed
-
- Hviid, L., and T. Staalsoe. 2004. Malaria immunity in infants: a special case of a general phenomenon? Trends Parasitol. 20:66–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

