2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells
- PMID: 15123744
- PMCID: PMC2211902
- DOI: 10.1084/jem.20031989
2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells
Abstract
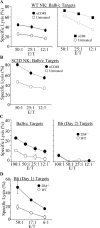
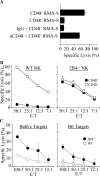
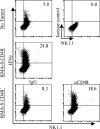
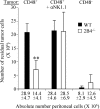
Natural killer (NK) cells are critical in the immune response to tumor cells, virally infected cells, and bone marrow allografts. 2B4 (CD244) is expressed on all NK cells and the ligand for 2B4, CD48, is expressed on hematopoietic cells. Cross-linking 2B4 on NK cells with anti-2B4 monoclonal antibody leads to NK cell activation in vitro. Therefore, 2B4 is considered to be an activating receptor. Surprisingly, we have found, using antibody-blocking and 2B4-deficient NK cells, that NK lysis of CD48(+) tumor and allogeneic targets is inhibited by 2B4 ligation. Interferon gamma production by NK cells is also inhibited. Using a peritoneal tumor clearance assay, it was found that 2B4(-/-) mice have increased clearance of CD48(+) tumor cells in vivo. Retroviral transduction of 2B4 was sufficient to restore inhibition in 2B4(-/-) primary NK cells. It was found that although mature NK cells express SH2D1A, in vitro-derived NK cells do not. However, both populations are inhibited by 2B4 ligation. This indicates that 2B4 inhibitory signaling occurs regardless of the presence of SH2D1A. These findings reveal a novel role for 2B4 as a non-major histocompatibility complex binding negative regulator of NK cells.
Figures



References
-
- Biron, C.A., K.B. Nguyen, G.C. Pien, L.P. Cousens, and T.P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189–220. - PubMed
-
- Lanier, L.L. 1995. The role of natural killer cells in transplantation. Curr. Opin. Immunol. 7:626–631. - PubMed
-
- Lanier, L.L. 1998. NK cell receptors. Annu. Rev. Immunol. 16:359–393. - PubMed
-
- McQueen, K.L., and P. Parham. 2002. Variable receptors controlling activation and inhibition of NK cells. Curr. Opin. Immunol. 14:615–621. - PubMed
-
- Lanier, L.L. 2001. On guard–activating NK cell receptors. Nat. Immunol. 2:23–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

