The structure of a thermophilic archaeal virus shows a double-stranded DNA viral capsid type that spans all domains of life
- PMID: 15123802
- PMCID: PMC419672
- DOI: 10.1073/pnas.0401773101
The structure of a thermophilic archaeal virus shows a double-stranded DNA viral capsid type that spans all domains of life
Abstract
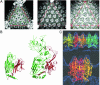
Of the three domains of life (Eukarya, Bacteria, and Archaea), the least understood is Archaea and its associated viruses. Many Archaea are extremophiles, with species that are capable of growth at some of the highest temperatures and extremes of pH of all known organisms. Phylogenetic rRNA-encoding DNA analysis places many of the hyperthermophilic Archaea (species with an optimum growth > or = 80 degrees C) at the base of the universal tree of life, suggesting that thermophiles were among the first forms of life on earth. Very few viruses have been identified from Archaea as compared to Bacteria and Eukarya. We report here the structure of a hyperthermophilic virus isolated from an archaeal host found in hot springs in Yellowstone National Park. The sequence of the circular double-stranded DNA viral genome shows that it shares little similarity to other known genes in viruses or other organisms. By comparing the tertiary and quaternary structures of the coat protein of this virus with those of a bacterial and an animal virus, we find conformational relationships among all three, suggesting that some viruses may have a common ancestor that precedes the division into three domains of life >3 billion years ago.
Figures


Comment in
-
Hot new virus, deep connections.Proc Natl Acad Sci U S A. 2004 May 18;101(20):7495-6. doi: 10.1073/pnas.0402151101. Epub 2004 May 11. Proc Natl Acad Sci U S A. 2004. PMID: 15138303 Free PMC article. No abstract available.
References
-
- Ackermann, H. W. (2001) Arch. Virol. 146, 843-857. - PubMed
-
- Rachel, R., Bettstetter, M., Hedlund, B. P., Haring, M., Kessler, A., Stetter, K. O. & Prangishvili, D. (2002) Arch. Virol. 147, 2419-2429. - PubMed
-
- Zillig, W., Arnold, H. P., Holz, I., Prangishvili, D., Schweier, A., Stedman, K., She, Q., Phan, H., Garrett, R. & Kristjansson, J. K. (1998) Extremophiles 2, 131-140. - PubMed
-
- Stetter, K. (1999) FEBS Lett. 452, 22-25. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

