The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry
- PMID: 15123804
- PMCID: PMC409938
- DOI: 10.1073/pnas.0401883101
The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry
Abstract
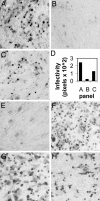
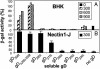
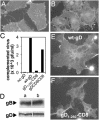
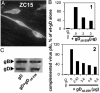
Entry of herpes simplex virus (HSV) 1 into cells requires the interaction of HSV gD with herpesvirus entry mediator or nectin1 receptors, and fusion with cell membrane mediated by the fusion glycoproteins gB, gH, and gL. We report that the gD ectodomain in soluble form (amino acids 1-305) was sufficient to rescue the infectivity of a gD-null HSV mutant, indicating that gD does not need to be anchored to the virion envelope to mediate entry. Entry mediated by soluble gD required, in addition to the receptor-binding sites contained within residues 1-250, a discrete downstream portion (amino acids 261-305), located proximal to the transmembrane segment in full-length gD. We named it as profusion domain. The pro-fusion domain was required for entry mediated by virion-bound gD, because its substitution with the corresponding region of CD8 failed to complement the infectivity of gD(-/+) HSV. Furthermore, a receptor-negative gD (gD(Delta6-259)) inhibited virus infectivity when coexpressed with wild-type gD; i.e., it acted as a dominant-negative gD mutant. The pro-fusion domain is proline-rich, which is characteristic of regions involved in protein-protein interactions. P291L-P292A substitutions diminished the gD capacity to complement gD(-/+) HSV infectivity. We propose that gD forms a tripartite complex with its receptor and, by way of the proline-rich pro-fusion domain, with the fusion glycoproteins, or with one of them. The tripartite complex would serve to recruit/activate the fusion glycoproteins and bring them from a fusion-inactive to a fusion-active state, such that they execute fusion of the virion envelope with cell membrane.
Figures




References
-
- Campadelli-Fiume, G., Cocchi, F., Menotti, L. & Lopez, M. (2000) Rev. Med. Virol. 10, 305-319. - PubMed
-
- Spear, P. G., Eisenberg, R. J. & Cohen, G. H. (2000) Virology 275, 1-8. - PubMed
-
- Montgomery, R. I., Warner, M. S., Lum, B. J. & Spear, P. G. (1996) Cell 87, 427-436. - PubMed
-
- Eberlé, F., Dubreuil, P., Mattei, M. G., Devilard, E. & Lopez, M. (1995) Gene 159, 267-272. - PubMed
-
- Lopez, M., Eberlé, F., Mattei, M. G., Gabert, J., Birg, F., Bardin, F., Maroc, C. & Dubreuil, P. (1995) Gene 155, 261-265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

