Malondialdehyde adducts in DNA arrest transcription by T7 RNA polymerase and mammalian RNA polymerase II
- PMID: 15123825
- PMCID: PMC409909
- DOI: 10.1073/pnas.0402252101
Malondialdehyde adducts in DNA arrest transcription by T7 RNA polymerase and mammalian RNA polymerase II
Abstract
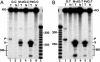
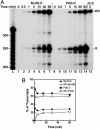
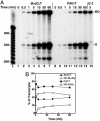
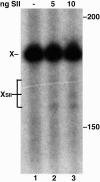
Malondialdehyde, a genotoxic byproduct of lipid peroxidation, reacts with guanine in DNA to form pyrimido[1,2-alpha]purin-10(3H)one (M(1)dG), the first endogenous DNA lesion found to be a target of nucleotide excision repair enzymes. A subpathway of nucleotide excision repair, transcription-coupled repair, is thought to occur when RNA polymerase (RNAP) is arrested at damage in transcribed DNA strands and might function for efficient removal of M(1)dG in active genes. Results presented here show that M(1)dG and its stable, exocyclic analog 1,N(2)-propanodeoxyguanine (PdG), arrest translocation of T7 RNAP and mammalian RNAPII when located in the transcribed strand of a DNA template. M(1)dG paired with thymine is exocyclic and poses a stronger block to transcription than the acyclic N(2)-(3-oxo-1-propenyl)-dG, formed upon cytosine-catalyzed opening of M(1)dG in duplex DNA. PdG is a complete block to RNAPII regardless of base pairing. The elongation factor TFIIS (SII) induces reversal and RNA transcript cleavage by RNAPII arrested at PdG. Thus, arrested RNAPII complexes may be stable at M(1)dG in cells and may resume transcription once the offending adduct is removed. The conclusion from this work is that malondialdehyde adducts in the transcribed strand of expressed genes are strong blocks to RNAPs and are targets for cellular transcription-coupled repair. If so, then M(1)dG, already known to be highly mutagenic in human cells, also may contribute to apoptosis in the developing tissues of individuals with Cockayne's syndrome, a hereditary disorder characterized by transcription-coupled repair deficiency.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

