Hydration and mobility of HO-(aq)
- PMID: 15123832
- PMCID: PMC409901
- DOI: 10.1073/pnas.0401696101
Hydration and mobility of HO-(aq)
Abstract
The hydroxide anion plays an essential role in many chemical and biochemical reactions. But a molecular-scale description of its hydration state, and hence also its transport, in water is currently controversial. The statistical mechanical quasichemical theory of solutions suggests that HO.[H2O]3(-) is the predominant species in the aqueous phase under standard conditions. This result agrees with recent spectroscopic studies on hydroxide water clusters and with the available thermodynamic hydration free energies. In contrast, a recent ab initio molecular dynamics simulation has suggested that HO.[H2O]4(-) is the only dominant aqueous solution species. We apply adiabatic ab initio molecular dynamics simulations and find good agreement with both the quasichemical theoretical predictions and experimental results. The present results suggest a picture that is simpler, more traditional, but with additional subtlety. These coordination structures are labile but the tricoordinate species is the prominent case. This conclusion is unaltered with changes in the electronic density functional. No evidence is found for rate-determining activated interconversion of a HO.[H2O]4(-) trap structure to HO.[H2O]3(-) mediating hydroxide transport. The view of HO- diffusion as the hopping of a proton hole has substantial validity, the rate depending largely on the dynamic disorder of the water hydrogen-bond network.
Figures
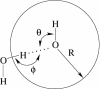


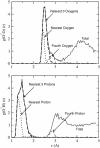
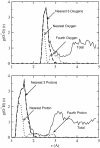
References
-
- Cecconi, F., Ghilardi, C. A., Ienco, A., Mariani, P., Mealli, C., Midollini, S., Orlandini, A. & Vacca, A. (2002) Inorg. Chem. 41, 4006-4017. - PubMed
-
- Asthagiri, D. & Pratt, L. R. (2003) Chem. Phys. Lett. 371, 613-619.
-
- Bernal, J. D. & Fowler, R. H. (1933) J. Chem. Phys. 1, 515-548.
-
- Eigen, M. (1964) Angew. Chem. Intl. Ed. 3, 1-72.
-
- Stillinger, F. H. (1978) in Theoretical Chemistry: Advances and Perspectives, eds. Eyring, H. & Henderson, D. (Academic, New York) Vol. 3, pp. 177-234.
LinkOut - more resources
Full Text Sources

