The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1
- PMID: 15123840
- PMCID: PMC404070
- DOI: 10.1073/pnas.0401139101
The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1
Abstract
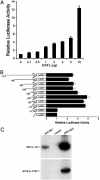
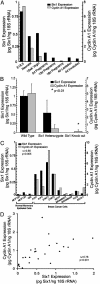
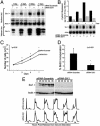
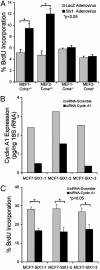
Homeobox genes constitute a large family of transcription factors that are essential during normal development and are often dysregulated in cancer. However, the molecular mechanisms by which homeobox genes influence cancer remain largely unknown. Here we show that the tissue-restricted cyclin A1 is a transcriptional target of the Six1 homeoprotein. Both genes are expressed in the embryonic but not the terminally differentiated mammary gland, and Six1-knockout mice show a dramatic reduction of cyclin A1 in the embryonic mammary gland. In addition, both genes are reexpressed in breast cancers. Six1 overexpression increases cyclin A1 mRNA levels and activity, cell proliferation, and tumor volume, whereas Six1 down-regulation decreases cyclin A1 mRNA levels and proliferation. Overexpression of Six1 in wild-type mouse embryonic fibroblasts, but not in knockout variants lacking the cyclin A1 gene, induces cell proliferation. Furthermore, inhibition of cyclin A1 in Six1-overexpressing mammary carcinoma cells decreases proliferation. Together these results demonstrate that cyclin A1 is required for the proliferative effect of Six1. We conclude that Six1 overexpression reinstates an embryonic pathway of proliferation in breast cancer by up-regulating cyclin A1.
Figures
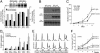
References
-
- Abate-Shen, C. (2002) Nat. Rev. Cancer 2, 777-785. - PubMed
-
- Ford, H. L. (1998) Cell Biol. Int. 22, 397-400. - PubMed
-
- Li, X., Perissi, V., Liu, F., Rose, D. W. & Rosenfeld, M. G. (2002) Science 297, 1180-1183. - PubMed
-
- Kawakami, K., Sato, S., Ozaki, H. & Ikeda, K. (2000) BioEssays 22, 616-626. - PubMed
-
- Relaix, F. & Buckingham, M. (1999) Genes Dev. 13, 3171-3178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

