Bacterial flagellin is a dominant antigen in Crohn disease
- PMID: 15124021
- PMCID: PMC398429
- DOI: 10.1172/JCI20295
Bacterial flagellin is a dominant antigen in Crohn disease
Abstract
Chronic intestinal inflammation, as seen in inflammatory bowel disease (IBD), results from an aberrant and poorly understood mucosal immune response to the microbiota of the gastrointestinal tract in genetically susceptible individuals. Here we used serological expression cloning to identify commensal bacterial proteins that could contribute to the pathogenesis of IBD. The dominant antigens identified were flagellins, molecules known to activate innate immunity via Toll-like receptor 5 (TLR5), and critical targets of the acquired immune system in host defense. Multiple strains of colitic mice had elevated serum anti-flagellin IgG2a responses and Th1 T cell responses to flagellin. In addition, flagellin-specific CD4(+) T cells induced severe colitis when adoptively transferred into naive SCID mice. Serum IgG to these flagellins, but not to the dissimilar Salmonella muenchen flagellin, was elevated in patients with Crohn disease, but not in patients with ulcerative colitis or in controls. These results identify flagellins as a class of immunodominant antigens that stimulate pathogenic intestinal immune reactions in genetically diverse hosts and suggest new avenues for the diagnosis and antigen-directed therapy of patients with IBD.
Figures

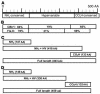
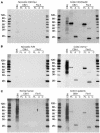




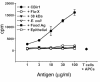

References
-
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. - PubMed
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3:521–533. - PubMed
-
- McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 2001;3:1–11. - PubMed
-
- Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 2003;15:396–401. - PubMed
-
- Akira S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003;15:5–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

