Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis
- PMID: 15124023
- PMCID: PMC398427
- DOI: 10.1172/JCI19930
Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis
Abstract
Pre-B cell colony-enhancing factor (PBEF) is a highly conserved 52-kDa protein, originally identified as a growth factor for early stage B cells. We show here that PBEF is also upregulated in neutrophils by IL-1beta and functions as a novel inhibitor of apoptosis in response to a variety of inflammatory stimuli. Induction of PBEF occurs 5-10 hours after LPS exposure. Prevention of PBEF translation with an antisense oligonucleotide completely abrogates the inhibitory effects of LPS, IL-1, GM-CSF, IL-8, and TNF-alpha on neutrophil apoptosis. Immunoreactive PBEF is detectable in culture supernatants from LPS-stimulated neutrophils, and a recombinant PBEF fusion protein inhibits neutrophil apoptosis. PBEF is also expressed in neutrophils from critically ill patients with sepsis in whom rates of apoptosis are profoundly delayed. Expression occurs at higher levels than those seen in experimental inflammation, and a PBEF antisense oligonucleotide significantly restores the normal kinetics of apoptosis in septic polymorphonuclear neutrophils. Inhibition of apoptosis by PBEF is associated with reduced activity of caspases-8 and -3, but not caspase-9. These data identify PBEF as a novel inflammatory cytokine that plays a requisite role in the delayed neutrophil apoptosis of clinical and experimental sepsis.
Figures
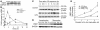
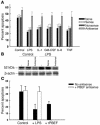

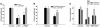

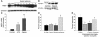
References
-
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. - PubMed
-
- Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. - PubMed
-
- Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. - PubMed
-
- Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 2000;343:1703–1714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

