Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae
- PMID: 15125783
- PMCID: PMC428576
- DOI: 10.1186/1471-2334-4-12
Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae
Abstract
Background: Despite its direct connection to the nasopharynx which harbors otitis media pathogens as part of its normal flora, the middle ear cavity is kept free of these bacteria by as yet unknown mechanisms. Respiratory mucosal epithelia, including those of the middle ear and eustachian tube, secrete antimicrobial effectors including lysozyme, lactoferrin and beta defensins-1 and -2. To elucidate the role of these innate immune molecules in the normal defense and maintenance of sterility of respiratory mucosa such as that of the middle ear, we assessed their effect on the respiratory pathogens nontypeable Haemophilus influenzae (NTHi) 12, Moraxella catarrhalis 035E, and Streptococcus pneumoniae 3, and 6B.
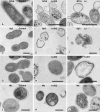
Methods: Two assay methods, the radial assay and the liquid broth assay, were employed for testing the antimicrobial activity of the molecules. This was done in order to minimize the possibility that the observed effects were artifacts of any single assay system employed. Also, transmission electron microscopy (TEM) was employed to evaluate the effect of antimicrobial innate immune molecules on OM pathogens. For the statistical analysis of the data, Student's t-test was performed.
Results: Results of the radial diffusion assay showed that beta defensin-2 was active against all four OM pathogens tested, while treatment with beta defensin-1 appeared to only affect M. catarrhalis. The radial assay results also showed that lysozyme was quite effective against S. pneumoniae 3 and 6B and was partially bacteriostatic/bactericidal against M. catarrhalis. Lysozyme however, appeared not to affect the growth of NTHi. Thus, lysozyme seems to have a more pronounced impact on the growth of the Gram-positive S. pneumoniae as compared to that of Gram-negative pathogens. Lactoferrin on the other hand, enhanced the growth of the bacteria tested. The results of the radial assays were confirmed using liquid broth assays for antimicrobial activity, and showed that lysozyme and beta defensin-2 could act synergistically against S. pneumoniae 6B. Moreover, in the liquid broth assay, beta defensin-1 showed a modest inhibitory effect on the growth of S. pneumoniae 6B. As assessed by ultrastructural analysis, lysozyme and beta defensin-2, and to a much lesser extent, beta defensin-1, appeared to be able to cause damage to the bacterial membranes.
Conclusions: Here we report that lysozyme and the beta defensins can inhibit the growth of clinical isolates of otitis media pathogens - namely NTHi strain 12, S. pneumoniae strains 3 and 6B and M. catarrhalis strain 035E - and cause ultrastructural damage to these pathogens. Moreover, we demonstrate that lysozyme and beta defensin-2 can act synergistically against S. pneumoniae. These findings are consistent with the concept that secreted antimicrobial peptides and other components of innate immunity constitute the first line of defense protecting host mucosal surfaces, including the tubotympanal (eustachian tube and middle ear cavity) mucosa, against pathogens.
Figures

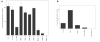
References
-
- Bluestone C, Klein J. Otits media in infants and children. 2. Philadelphia: W.B. Saunders Company; 1995.
-
- Maxson S, Yamauchi T. Acute otitis media. Pediatr Rev. 1996;17:191–195. quiz 196. - PubMed
-
- Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, Rixter DD, Kai H, Andalibi A, Linthicum F, et al. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem. 2002;277:17263–17270. doi: 10.1074/jbc.M112190200. - DOI - PubMed
-
- Rosenfeld R, Bluestone C. Evidence-Based Otitis Media. Saint Louis: BC Decker Inc. 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

