Characterization of monospecies biofilm formation by Helicobacter pylori
- PMID: 15126474
- PMCID: PMC400600
- DOI: 10.1128/JB.186.10.3124-3132.2004
Characterization of monospecies biofilm formation by Helicobacter pylori
Abstract
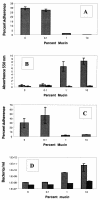
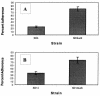
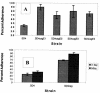
As all bacteria studied to date, the gastric pathogen Helicobacter pylori has an alternate lifestyle as a biofilm. H. pylori forms biofilms on glass surfaces at the air-liquid interface in stationary or shaking batch cultures. By light microscopy, we have observed attachment of individual, spiral H. pylori to glass surfaces, followed by division to form microcolonies, merging of individual microcolonies, and growth in the third dimension. Scanning electron micrographs showed H. pylori arranged in a matrix on the glass with channels for nutrient flow, typical of other bacterial biofilms. To understand the importance of biofilms to the H. pylori life cycle, we tested the effect of mucin on biofilm formation. Our results showed that 10% mucin greatly increased the number of planktonic H. pylori while not affecting biofilm bacteria, resulting in a decline in percent adherence to the glass. This suggests that in the mucus-rich stomach, H. pylori planktonic growth is favored over biofilm formation. We also investigated the effect of specific mutations in several genes, including the quorum-sensing gene, luxS, and the cagE type IV secretion gene. Both of these mutants were found to form biofilms approximately twofold more efficiently than the wild type in both assays. These results indicate the relative importance of these genes to the production of biofilms by H. pylori and the selective enhancement of planktonic growth in the presence of gastric mucin.
Figures


References
-
- Bunn, J. E. G., W. G. MacKay, J. E. Thomas, D. C. Reid, and L. T. Weaver. 2002. Detection of Helicobacter pylori DNA in drinking water biofilms: implications for transmission in early life. Lett. Appl. Microbiol. 34:450-454. - PubMed
-
- Cole, S. P., V. F. Kharitonov, and D. G. Guiney. 1999. Effect of nitric oxide on Helicobacter pylori morphology. J. Infect. Dis. 180:1713-1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

