A phase I trial of idiotypic vaccination with HMFG1 in ovarian cancer
- PMID: 15127236
- PMCID: PMC11032936
- DOI: 10.1007/s00262-004-0522-z
A phase I trial of idiotypic vaccination with HMFG1 in ovarian cancer
Abstract
Introduction: An extended phase I trial was conducted in a total of 26 patients with ovarian cancer. The objectives were to assess the safety and tolerability of idiotypic vaccination using the murine monoclonal antibody HMFG1 (anti-MUC1), and to develop robust assays to monitor humoral immune responses generated against either the antibody or MUC1.
Material and methods: All patients had undergone standard debulking surgery (where appropriate) and at least one regimen of platinum-based chemotherapy. Eligibility criteria included: (a) residual disease at the end of chemotherapy, (b) relapsed disease, and (c) pathologically confirmed second complete remission following salvage chemotherapy. Patients received a priming dose of 25 mg of HMFG1 either intravenously or intraperitoneally, followed by up to six intradermal doses of HMFG1 in 10% Alhydrogel at intervals of 1 month. The three dose levels were 0.5 mg, 1 mg and 5 mg. We devised modifications of published protocols for the measurement of anti-idiotypic and anti-MUC1 antibody responses and also extended the use of the IAsys resonant mirror biosensor to measure the kinetics of the idiotypic network response in these patients.
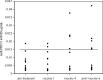
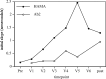
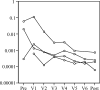
Results: There were no serious adverse events at any dose level. The trial confirmed that all doses could be administered safely with minimal toxicity. No clinical responses were seen in patients with evaluable disease. ELISA for anti-idiotypic antibodies (Ab2) showed significant levels in patients who completed the protocol. There were no significant differences in the levels of Ab2 generated by the different doses of antibody. These results were confirmed by biosensor assays for Ab2, which also showed affinity maturation of the Ab2 response as patients progressed through the vaccination protocol. Biosensor assays also demonstrated no difference in the affinity of Ab2 generated by different booster doses of HMFG1. ELISA for anti-MUC1 antibodies showed less consistent results, with very small but statistically significant rises in anti-MUC1 signals seen in 38% of patients who completed the vaccination regimen.
Discussion: The clinical endpoints of safety and tolerability were met. The assays developed for this project have shown reproducibility and may provide surrogate endpoints to assess vaccination for future trials. The use of similar biosensors may be of particular relevance for monitoring of humoral immune responses in other vaccine trials. The low levels of anti-MUC1 antibodies generated may correspond with the lack of clinical efficacy in the few patients with evaluable disease.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

