Heat shock protein 70 / MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo
- PMID: 15127237
- PMCID: PMC11034208
- DOI: 10.1007/s00262-004-0536-6
Heat shock protein 70 / MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo
Abstract
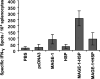
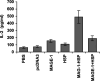
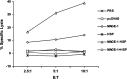
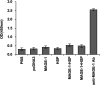
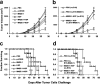
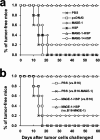
The cancer-testis antigen encoded by the MAGE-1 gene is an attractive antigen in tumor immunotherapy because it can be processed as a foreign antigen by the immune system and generate tumor-specific cellular immune response in vivo. However, increase of the potency of MAGE-1 DNA vaccines is still needed. The high degree of sequence homology and intrinsic immunogenicity of heat shock protein 70 (HSP70) have prompted the suggestion that HSP70 might have immunotherapeutic potential, as HSP70 purified from malignant and virally infected cells can transfer and deliver antigenic peptides to antigen-presenting cells to elicit peptide-specific immunity. In this research, we evaluated the enhancement of linkage of Mycobacterium tuberculosis HSP70 to MAGE-1 gene of the potency of antigen-specific immunity elicited by naked DNA vaccines. We found that vaccines containing MAGE-1-HSP70 fusion genes enhanced the frequency of MAGE-1-specific cytotoxic T cells in contract to vaccines containing the MAGE-1 gene alone. More importantly, the fusion converted a less effective DNA vaccine into one with significant potency against established MAGE-1-expressing tumors. These results indicate that linkage of HSP70 to MAGE-1 gene may greatly enhance the potency of DNA vaccines, and generate specific antitumor immunity against MAGE-1-expressing tumors.
Figures

Similar articles
-
Heat shock protein 70/MAGE-3 fusion protein vaccine can enhance cellular and humoral immune responses to MAGE-3 in vivo.Cancer Immunol Immunother. 2005 Sep;54(9):907-14. doi: 10.1007/s00262-004-0660-3. Epub 2005 Mar 9. Cancer Immunol Immunother. 2005. PMID: 15756604 Free PMC article.
-
Fusion of Hsp70 to Mage-a1 enhances the potency of vaccine-specific immune responses.J Transl Med. 2013 Dec 5;11:300. doi: 10.1186/1479-5876-11-300. J Transl Med. 2013. PMID: 24314011 Free PMC article.
-
A novel DNA vaccine constructed by heat shock protein 70 and melanoma antigen-encoding gene 3 against tumorigenesis.Indian J Exp Biol. 2010 May;48(5):436-43. Indian J Exp Biol. 2010. PMID: 20795360
-
DNA vaccine for cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3153-64. doi: 10.4161/21645515.2014.980686. Hum Vaccin Immunother. 2014. PMID: 25625927 Free PMC article. Review.
-
Could mycobacterial Hsp70-containing fusion protein lead the way to an affordable therapeutic cancer vaccine?Expert Rev Vaccines. 2015 Mar;14(3):435-46. doi: 10.1586/14760584.2015.979797. Epub 2014 Dec 13. Expert Rev Vaccines. 2015. PMID: 25496347 Review.
Cited by
-
Heat shock protein 70/MAGE-3 fusion protein vaccine can enhance cellular and humoral immune responses to MAGE-3 in vivo.Cancer Immunol Immunother. 2005 Sep;54(9):907-14. doi: 10.1007/s00262-004-0660-3. Epub 2005 Mar 9. Cancer Immunol Immunother. 2005. PMID: 15756604 Free PMC article.
-
The preparation of HL-60 cells vaccine expressing BCG heat shock protein 70 and detection of its immunogenicity in vitro.Hum Vaccin Immunother. 2012 Oct;8(10):1376-81. doi: 10.4161/hv.21321. Epub 2012 Aug 16. Hum Vaccin Immunother. 2012. PMID: 22894947 Free PMC article.
-
In vivo enhancement of the MAGE-specific cellular immune response by a recombinant MAGE1-MAGE3-TBHSP70 tumor vaccine.Cancer Cell Int. 2016 Jun 17;16:45. doi: 10.1186/s12935-016-0317-2. eCollection 2016. Cancer Cell Int. 2016. PMID: 27330408 Free PMC article.
-
Fusion of Hsp70 to Mage-a1 enhances the potency of vaccine-specific immune responses.J Transl Med. 2013 Dec 5;11:300. doi: 10.1186/1479-5876-11-300. J Transl Med. 2013. PMID: 24314011 Free PMC article.
-
Plasmid DNA for Therapeutic Applications in Cancer.Pharmaceutics. 2022 Sep 3;14(9):1861. doi: 10.3390/pharmaceutics14091861. Pharmaceutics. 2022. PMID: 36145609 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

