TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic beta-cells: different behaviour of granule motion between normal and Goto-Kakizaki diabetic rat beta-cells
- PMID: 15128287
- PMCID: PMC1133756
- DOI: 10.1042/BJ20040434
TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic beta-cells: different behaviour of granule motion between normal and Goto-Kakizaki diabetic rat beta-cells
Abstract
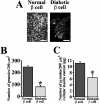
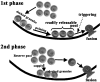
We imaged and analysed the motion of single insulin secretory granules near the plasma membrane in live pancreatic beta-cells, from normal and diabetic Goto-Kakizaki (GK) rats, using total internal reflection fluorescence microscopy (TIRFM). In normal rat primary beta-cells, the granules that were fusing during the first phase originate from previously docked granules, and those during the second phase originate from 'newcomers'. In diabetic GK rat beta-cells, the number of fusion events from previously docked granules were markedly reduced, and, in contrast, the fusion from newcomers was still preserved. The dynamic change in the number of docked insulin granules showed that, in GK rat beta-cells, the total number of docked insulin granules was markedly decreased to 35% of the initial number after glucose stimulation. Immunohistochemistry with anti-insulin antibody observed by TIRFM showed that GK rat beta-cells had a marked decline of endogenous insulin granules docked to the plasma membrane. Thus our results indicate that the decreased number of docked insulin granules accounts for the impaired insulin release during the first phase of insulin release in diabetic GK rat beta-cells.
Figures


References
-
- Lang T., Wacker I., Steyer J., Kaether C., Wunderlich I., Soldati T., Gerdes H. H., Almers W. Ca2+-triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent wave microscopy. Neuron. 1997;18:857–863. - PubMed
-
- Axelrod D. Total internal reflection fluorescent microscopy in cell biology. Traffic. 2001;2:764–774. - PubMed
-
- Ohara-Imaizumi M., Nakamichi Y., Tanaka T., Ishida H., Nagamatsu S. Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy. J. Biol. Chem. 2002;277:3805–3808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

