Isolation, characterization, and U(VI)-reducing potential of a facultatively anaerobic, acid-resistant Bacterium from Low-pH, nitrate- and U(VI)-contaminated subsurface sediment and description of Salmonella subterranea sp. nov
- PMID: 15128557
- PMCID: PMC404420
- DOI: 10.1128/AEM.70.5.2959-2965.2004
Isolation, characterization, and U(VI)-reducing potential of a facultatively anaerobic, acid-resistant Bacterium from Low-pH, nitrate- and U(VI)-contaminated subsurface sediment and description of Salmonella subterranea sp. nov
Abstract
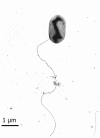
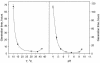
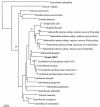
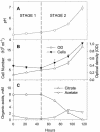
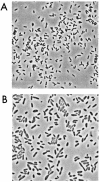
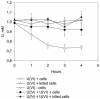
A facultatively anaerobic, acid-resistant bacterium, designated strain FRCl, was isolated from a low-pH, nitrate- and U(VI)-contaminated subsurface sediment at site FW-024 at the Natural and Accelerated Bioremediation Research Field Research Center in Oak Ridge, Tenn. Strain FRCl was enriched at pH 4.5 in minimal medium with nitrate as the electron acceptor, hydrogen as the electron donor, and acetate as the carbon source. Clones with 16S ribosomal DNA (rDNA) sequences identical to the sequence of strain FRCl were also detected in a U(VI)-reducing enrichment culture derived from the same sediment. Cells of strain FRCl were gram-negative motile regular rods 2.0 to 3.4 micro m long and 0.7 to 0.9 microm in diameter. Strain FRCl was positive for indole production, by the methyl red test, and for ornithine decarboxylase; it was negative by the Voges-Proskauer test (for acetylmethylcarbinol production), for urea hydrolysis, for arginine dihydrolase, for lysine decarboxylase, for phenylalanine deaminase, for H(2)S production, and for gelatin hydrolysis. Strain FRCl was capable of using O(2), NO(3)(-), S(2)O(3)(2-), fumarate, and malate as terminal electron acceptors and of reducing U(VI) in the cell suspension. Analysis of the 16S rDNA sequence of the isolate indicated that this strain was 96.4% similar to Salmonella bongori and 96.3% similar to Enterobacter cloacae. Physiological and phylogenetic analyses suggested that strain FRCl belongs to the genus Salmonella and represents a new species, Salmonella subterranea sp. nov.
Figures
Similar articles
-
Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI).Appl Environ Microbiol. 2003 Dec;69(12):7467-79. doi: 10.1128/AEM.69.12.7467-7479.2003. Appl Environ Microbiol. 2003. PMID: 14660400 Free PMC article.
-
Geobacter daltonii sp. nov., an Fe(III)- and uranium(VI)-reducing bacterium isolated from a shallow subsurface exposed to mixed heavy metal and hydrocarbon contamination.Int J Syst Evol Microbiol. 2010 Mar;60(Pt 3):546-553. doi: 10.1099/ijs.0.010843-0. Epub 2009 Aug 4. Int J Syst Evol Microbiol. 2010. PMID: 19654355
-
Geobacter uraniireducens sp. nov., isolated from subsurface sediment undergoing uranium bioremediation.Int J Syst Evol Microbiol. 2008 May;58(Pt 5):1075-8. doi: 10.1099/ijs.0.65377-0. Int J Syst Evol Microbiol. 2008. PMID: 18450691
-
Bacillus subterraneus sp. nov., an iron- and manganese-reducing bacterium from a deep subsurface Australian thermal aquifer.Int J Syst Evol Microbiol. 2002 May;52(Pt 3):869-874. doi: 10.1099/00207713-52-3-869. Int J Syst Evol Microbiol. 2002. PMID: 12054251
-
Geobacter lovleyi sp. nov. strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium.Appl Environ Microbiol. 2006 Apr;72(4):2775-82. doi: 10.1128/AEM.72.4.2775-2782.2006. Appl Environ Microbiol. 2006. PMID: 16597982 Free PMC article.
Cited by
-
Isolation and characterization of organophosphorus phosphatases from Bacillus thuringiensis MB497 capable of degrading Chlorpyrifos, Triazophos and Dimethoate.Heliyon. 2020 Jul 1;6(7):e04221. doi: 10.1016/j.heliyon.2020.e04221. eCollection 2020 Jul. Heliyon. 2020. PMID: 32642578 Free PMC article.
-
Evolution of Salmonella nomenclature: a critical note.Folia Microbiol (Praha). 2011 Nov;56(6):497-503. doi: 10.1007/s12223-011-0075-4. Epub 2011 Nov 4. Folia Microbiol (Praha). 2011. PMID: 22052214
-
Isolation and physiology of bacteria from contaminated subsurface sediments.Appl Environ Microbiol. 2010 Nov;76(22):7413-9. doi: 10.1128/AEM.00376-10. Epub 2010 Sep 24. Appl Environ Microbiol. 2010. PMID: 20870785 Free PMC article.
-
The Changing Face of the Family Enterobacteriaceae (Order: "Enterobacterales"): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes.Clin Microbiol Rev. 2021 Feb 24;34(2):e00174-20. doi: 10.1128/CMR.00174-20. Print 2021 Mar 17. Clin Microbiol Rev. 2021. PMID: 33627443 Free PMC article. Review.
-
Embracing Diversity: Differences in Virulence Mechanisms, Disease Severity, and Host Adaptations Contribute to the Success of Nontyphoidal Salmonella as a Foodborne Pathogen.Front Microbiol. 2019 Jun 26;10:1368. doi: 10.3389/fmicb.2019.01368. eCollection 2019. Front Microbiol. 2019. PMID: 31316476 Free PMC article. Review.
References
-
- Abdelouas, A., Y. Lu, W. Lutze, and H. E. Nuttall. 1998. Reduction of U(VI) to U(IV) by indigenous bacteria in contaminated ground water. J. Contam. Hydrol. 35:217-233.
-
- Abdelouas, A., W. Lutze, W. Gong, E. H. Nuttall, B. A. Strietelmeier, and B. J. Travis. 2000. Biological reduction of uranium in groundwater and subsurface soil. Sci. Total Environ. 250:21-35. - PubMed
-
- Anderson, R. T., and D. R. Lovley. 2002. Microbial redox interactions with uranium: an environmental perspective, p. 205-223. In M. J. Keith-Roach and F. R. Livens (ed.), Interactions of microorganisms with radionuclides. Elsevier, Amsterdam, The Netherlands.
-
- Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernard, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

