In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy
- PMID: 15128753
- PMCID: PMC2826362
- DOI: 10.1152/jn.00234.2004
In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy
Abstract
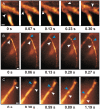
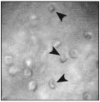
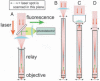
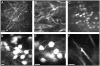
One of the major limitations in the current set of techniques available to neuroscientists is a dearth of methods for imaging individual cells deep within the brains of live animals. To overcome this limitation, we developed two forms of minimally invasive fluorescence microendoscopy and tested their abilities to image cells in vivo. Both one- and two-photon fluorescence microendoscopy are based on compound gradient refractive index (GRIN) lenses that are 350-1,000 microm in diameter and provide micron-scale resolution. One-photon microendoscopy allows full-frame images to be viewed by eye or with a camera, and is well suited to fast frame-rate imaging. Two-photon microendoscopy is a laser-scanning modality that provides optical sectioning deep within tissue. Using in vivo microendoscopy we acquired video-rate movies of thalamic and CA1 hippocampal red blood cell dynamics and still-frame images of CA1 neurons and dendrites in anesthetized rats and mice. Microendoscopy will help meet the growing demand for in vivo cellular imaging created by the rapid emergence of new synthetic and genetically encoded fluorophores that can be used to label specific brain areas or cell classes.
Figures




References
-
- Akaaboune M, Grady RM, Turney S, Sanes JR, Lichtman JW. Neurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands. Neuron. 2002;34:865–876. - PubMed
-
- Anandh B, Mohanty A, Sampath S, Praharaj SS, Kolluri S. Endoscopic approach to intraventricular cysticercal lesions. Minim Invasive Neurosurg. 2001;44:194–196. - PubMed
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. - PubMed
-
- Beaurepaire E, Oheim M, Mertz J. Ultra-deep two-photon fluorescence excitation in turbid media. Opt Commun. 2001;188:25–29.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

