Four classes of intercellular channels between glial cells in the CNS
- PMID: 15128845
- PMCID: PMC6729442
- DOI: 10.1523/JNEUROSCI.3303-03.2004
Four classes of intercellular channels between glial cells in the CNS
Abstract
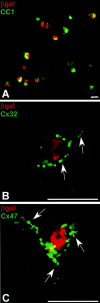
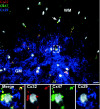
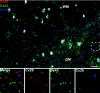
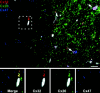
Astrocytes form extensive gap junctions with other astrocytes and with oligodendrocytes. Junctional communication between CNS glia is likely of critical importance because loss of the gap junction channel-forming proteins, connexins Cx32 and Cx47, result in severe demyelination. However, CNS glia express at least six connexins, and the cellular origins and relationships of these proteins have not been determined. We produced a Cx29 reporter mouse in which the connexin coding sequence was replaced with a histological marker, which was used to demonstrate that Cx29, Cx32, and Cx47 are expressed specifically in oligodendrocytes. To determine the relationships between astrocyte and oligodendrocyte connexins, we used double- and triple-immunofluorescence microscopy using semithin sections (<1 microm) of adult mouse spinal cord. Astrocytes form two distinct classes of gap junctions with each other; those composed of Cx26 and those composed of Cx43 and Cx30. In addition, astrocytes establish two classes of intercellular channels with oligodendrocytes, heterotypic Cx26-Cx32 channels and heterotypic Cx30/Cx43-Cx47 channels that may also be heteromeric. In contrast, Cx29 does not colocalize with any of the other five connexins. The data provide the first in vivo demonstration of heterotypic intercellular channels and reveal an unexpected complexity in the composition of glial gap junctions.
Figures






References
-
- Belliveau DJ, Naus CCG (1994) Cortical type 2 astrocytes are not dye coupled nor do they express the major gap junction genes found in the central nervous system. Glia 12: 24–34. - PubMed
-
- Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH (1993) Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262: 2039–2042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
