Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins
- PMID: 15128846
- PMCID: PMC6729446
- DOI: 10.1523/JNEUROSCI.5227-03.2004
Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins
Abstract
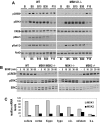
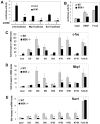
Activation of the transcription factor cAMP response element-binding protein (CREB) by neurotrophins is believed to regulate the survival, differentiation, and maturation of neurons in the CNS and PNS. Although phosphorylation of Ser133 is critical for the expression of CREB-regulated genes, the identity of neurotrophin-regulated Ser133 kinases has remained controversial. We show here that neurotrophin-induced CREB phosphorylation in CNS neurons depends exclusively on the extracellular signal-regulated kinase 1/2-activated kinase mitogen- and stress-activated protein kinase 1 (MSK1). Small interfering RNA directed against ribosomal S6 kinase 1 (RSK1) and RSK2 reduced phosphorylation of a RSK substrate but did not effect CREB-dependent transcription. However, expression of a selective inhibitory MSK1 mutant markedly attenuated BDNF-stimulated CREB phosphorylation and CREB-mediated transcription. Moreover, the ability of neurotrophins to stimulate CREB phosphorylation was abolished in CNS neurons from MSK1 knock-out mice. Consistent with a role for MSK1 in Ser133 phosphorylation, neurotrophin-induced expression of CREB-regulated genes was attenuated in MSK-deficient neurons. These results indicate that MSK1 is the major neurotrophin-activated Ser133 kinase in CNS neurons.
Figures




References
-
- Arthur JS, Cohen P (2000) MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett 482: 44–48. - PubMed
-
- Bonni A, Ginty DD, Dudek H, Greenberg ME (1995) Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci 6: 168–183. - PubMed
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362. - PubMed
-
- Bruning JC, Gillette JA, Zhao Y, Bjorbaeck C, Kotzka J, Knebel B, Avci H, Hanstein B, Lingohr P, Moller DE, Krone W, Kahn CR, Muller-Wieland D (2000) Ribosomal subunit kinase-2 is required for growth factor-stimulated transcription of the c-Fos gene. Proc Natl Acad Sci USA 97: 2462–2467. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
