Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb
- PMID: 15128852
- PMCID: PMC6729436
- DOI: 10.1523/JNEUROSCI.5570-03.2004
Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb
Abstract
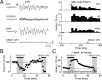
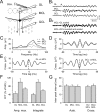
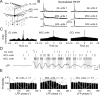
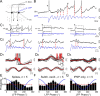
Olfactory stimuli have been known for a long time to elicit oscillations in olfactory brain areas. In the olfactory bulb (OB), odors trigger synchronous oscillatory activity that is believed to arise from the coherent and rhythmic discharges of large numbers of neurons. These oscillations are known to take part in encoding of sensory information before their transfer to higher subcortical and cortical areas. To characterize the cellular mechanisms underlying gamma (30-80 Hz) local field potential (LFP) oscillations, we simultaneously recorded multiunit discharges, intracellular responses, and LFP in rat OB slices. We showed that a single and brief electrical stimulation of olfactory nerve elicited LFP oscillations in the mitral cell body layer lasting >1 sec. Both action potentials and subthreshold oscillations of mitral/tufted cells, the bulbar output neurons, were precisely synchronized with LFP oscillations. This synchronization arises from the interaction between output neurons and granule cells, the main population of local circuit inhibitory interneurons, through dendrodendritic synapses. Interestingly enough, the synchronization exerted by reciprocal synaptic interactions did not require action potentials initiated in granule cell somata. Finally, local application of a GABA(A) receptor antagonist at the mitral cell and external plexiform layers confirmed the exclusive role of the granule cell reciprocal synapses in generating the evoked oscillations. We concluded that interneurons located in the granule cell layer generate synaptic activity capable of synchronizing activity of output neurons by interacting with both their subthreshold and spiking activity.
Figures


References
-
- Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433–457. - PubMed
-
- Becker CJ, Freeman WJ (1968) Prepyriform electrical activity after loss of peripheral or central input, or both. Physiol Behav 3: 597–599.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
