Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats
- PMID: 15128853
- PMCID: PMC6729440
- DOI: 10.1523/JNEUROSCI.0529-04.2004
Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats
Abstract
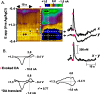
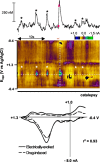
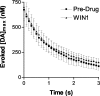
Dopaminergic neurotransmission has been highly implicated in the reinforcing properties of many substances of abuse, including marijuana. Cannabinoids activate ventral tegmental area dopaminergic neurons, the main ascending projections of the mesocorticolimbic dopamine system, and change their spiking pattern by increasing the number of impulses in a burst and elevating the frequency of bursts. Although they also increase time-averaged striatal dopamine levels for extended periods of time, little is known about the temporal structure of this change. To elucidate this, fast-scan cyclic voltammetry was used to monitor extracellular dopamine in the nucleus accumbens of freely moving rats with subsecond timescale resolution. Intravenous administration of the central cannabinoid (CB1) receptor agonist, R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-(1-naphthalenyl) methanone mesylate, dose-dependently produced catalepsy, decreased locomotion, and reduced the amplitude of electrically evoked dopamine release while markedly increasing the frequency of detected (nonstimulated) dopamine concentration transients. The CB1 receptor antagonist [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide] reversed and prevented all agonist-induced effects but did not show effects on dopamine release when injected alone. These data demonstrate that doses of a cannabinoid agonist known to increase burst firing produce ongoing fluctuations in extracellular dopamine on a previously unrecognized temporal scale in the nucleus accumbens.
Figures





Similar articles
-
Cannabinoid modulation of electrically evoked pH and oxygen transients in the nucleus accumbens of awake rats.J Neurochem. 2006 May;97(4):1145-54. doi: 10.1111/j.1471-4159.2006.03860.x. J Neurochem. 2006. PMID: 16686693
-
Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure.J Neurosci. 2003 Jun 15;23(12):4815-20. doi: 10.1523/JNEUROSCI.23-12-04815.2003. J Neurosci. 2003. PMID: 12832502 Free PMC article.
-
Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens.Neurochem Int. 2009 Jun;54(7):452-7. doi: 10.1016/j.neuint.2009.01.017. Epub 2009 Feb 6. Neurochem Int. 2009. PMID: 19428788
-
Endocannabinoid-dependent modulation of phasic dopamine signaling encodes external and internal reward-predictive cues.Front Psychiatry. 2014 Sep 1;5:118. doi: 10.3389/fpsyt.2014.00118. eCollection 2014. Front Psychiatry. 2014. PMID: 25225488 Free PMC article. Review.
-
Rapid Dopamine Release in Freely Moving Rats.In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 2. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 2. PMID: 21204389 Free Books & Documents. Review.
Cited by
-
Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants.Front Neurosci. 2016 Apr 19;10:153. doi: 10.3389/fnins.2016.00153. eCollection 2016. Front Neurosci. 2016. PMID: 27147945 Free PMC article. Review.
-
Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices.ACS Chem Neurosci. 2013 May 15;4(5):693-703. doi: 10.1021/cn400026v. Epub 2013 May 6. ACS Chem Neurosci. 2013. PMID: 23581570 Free PMC article. Review.
-
Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions.J Neurosci. 2016 Oct 5;36(40):10230-10238. doi: 10.1523/JNEUROSCI.1712-16.2016. J Neurosci. 2016. PMID: 27707960 Free PMC article. Review.
-
Methamphetamine-induced neurotoxicity disrupts pharmacologically evoked dopamine transients in the dorsomedial and dorsolateral striatum.Neurotox Res. 2014 Aug;26(2):152-67. doi: 10.1007/s12640-014-9459-y. Epub 2014 Feb 22. Neurotox Res. 2014. PMID: 24562969 Free PMC article.
-
Cannabinoid transmission in the prelimbic cortex bidirectionally controls opiate reward and aversion signaling through dissociable kappa versus μ-opiate receptor dependent mechanisms.J Neurosci. 2013 Sep 25;33(39):15642-51. doi: 10.1523/JNEUROSCI.1686-13.2013. J Neurosci. 2013. PMID: 24068830 Free PMC article.
References
-
- Budygin EA, Phillips PEM, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM (2001) Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther 297: 27–34. - PubMed
-
- Chen JP, Paredes W, Lowinson JH, Gardner EL (1991) Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett 129: 136–180. - PubMed
-
- Cheer JF, Marsden CA, Kendall DA, Mason R (2000a) Lack of response suppression follows repeated ventral tegmental cannabinoid administration: an in vitro electrophysiological study. Neuroscience 99: 661–667. - PubMed
-
- Cheer JF, Kendall DA, Marsden CA (2000b) Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 151: 25–30. - PubMed
-
- Cheer JF, Kendall DA, Mason R, Marsden CA (2003) Differential cannabinoid-induced electrophysiological effects in rat ventral tegmentum. Neuropharmacology 44: 633–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
