Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons
- PMID: 15128854
- PMCID: PMC6729450
- DOI: 10.1523/JNEUROSCI.0348-04.2004
Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons
Abstract
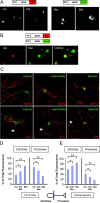
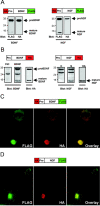
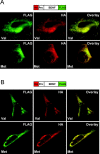
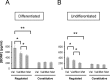
Brain-derived neurotrophic factor (BDNF) plays a critical role in nervous system and cardiovascular development and function. Recently, a common single nucleotide polymorphism in the bdnf gene, resulting in a valine to methionine substitution in the prodomain (BDNF(Met)), has been shown to lead to memory impairment and susceptibility to neuropsychiatric disorders in humans heterozygous for the variant BDNF. When expressed by itself in hippocampal neurons, less BDNF(Met) is secreted in an activity-dependent manner. The nature of the cellular defect when both BDNF(Met) and wild-type BDNF (BDNF(Val)) are present in the same cell is not known. Given that this is the predominant expression profile in humans, we examined the effect of coexpressed BDNF(Met) on BDNF(Val) intracellular trafficking and processing. Our data indicate that abnormal trafficking of BDNF(Met) occurred only in neuronal and neurosecretory cells and that BDNF(Met) could alter the intracellular distribution and activity-dependent secretion of BDNF(Val). We determined that, when coexpressed in the same cell, approximately 70% of the variant BDNF forms BDNF(Val).BDNF(Met) heterodimers, which are inefficiently sorted into secretory granules resulting in a quantitative decreased secretion. Finally, we determined the form of BDNF secreted in an activity-dependent manner and observed no differences in the forms of BDNF(Met) or the BDNF(Val).BDNF(Met) heterodimer compared with BDNF(Val). Together, these findings indicate that components of the regulated secretory machinery interacts specifically with a signal in the BDNF prodomain and that perturbations in BDNF trafficking may lead to selective impairment in CNS function.
Figures


References
-
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309. - PubMed
-
- Donovan M, Lin M, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez C, Rafii S, Hempstead B (2000) Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 127: 4531–4540. - PubMed
-
- Egan M, Kojima M, Callicott J, Goldberg T, Kolachana B, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger D (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
