Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat
- PMID: 15128861
- PMCID: PMC6729432
- DOI: 10.1523/JNEUROSCI.5560-03.2004
Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat
Abstract
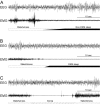
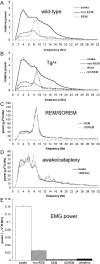
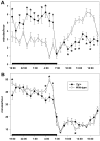
The sleep disorder narcolepsy has been linked to loss of hypothalamic neurons producing the orexin (hypocretin) neuropeptides. Here, we report the generation of transgenic rats expressing a human ataxin-3 fragment with an elongated polyglutamyl stretch under control of the human prepro-orexin promoter (orexin/ataxin-3 rats). At 17 weeks of age, the transgenic rats exhibited postnatal loss of orexin-positive neurons in the lateral hypothalamus, and orexin-containing projections were essentially undetectable. The loss of orexin production resulted in the expression of a phenotype with fragmented vigilance states, a decreased latency to rapid eye movement (REM) sleep and increased REM sleep time during the dark active phase. Wakefulness time was also reduced during the dark phase, and this effect was concentrated at the photoperiod boundaries. Direct transitions from wakefulness to REM sleep, a defining characteristic of narcolepsy, occurred frequently. Brief episodes of muscle atonia and postural collapse resembling cataplexy were also noted while rats maintained the electroencephalographic characteristics of wakefulness. These findings indicate that the orexin/ataxin-3 transgenic rat could provide a useful model of human narcolepsy.
Figures


References
-
- Aldrich MS (1992) Narcolepsy. Neurology 42[Suppl 6]: 34–43. - PubMed
-
- Bassetti C, Aldrich MS (1996) Narcolepsy. Neurol Clin 14: 545–571. - PubMed
-
- Beuckmann CT, Yanagisawa M (2002) Orexins: from neuropeptides to energy homeostasis and sleep/wake regulation. J Mol Med 80: 329–342. - PubMed
-
- Billiard M (1985) Narcolepsy–clinical features and aetiology. Ann Clin Res 17: 220–226. - PubMed
-
- Brown RE, Sergeeva O, Eriksson KS, Haas HL (2001) Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40: 457–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
