Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase
- PMID: 15128933
- PMCID: PMC419671
- DOI: 10.1073/pnas.0402490101
Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase
Abstract
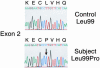
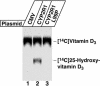
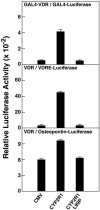
The synthesis of bioactive vitamin D requires hydroxylation at the 1 alpha and 25 positions by cytochrome P450 enzymes in the kidney and liver, respectively. The mitochondrial enzyme CYP27B1 catalyzes 1 alpha-hydroxylation in the kidney but the identity of the hepatic 25-hydroxylase has remained unclear for >30 years. We previously identified the microsomal CYP2R1 protein as a potential candidate for the liver vitamin D 25-hydroxylase based on the enzyme's biochemical properties, conservation, and expression pattern. Here, we report a molecular analysis of a patient with low circulating levels of 25-hydroxyvitamin D and classic symptoms of vitamin D deficiency. This individual was found to be homozygous for a transition mutation in exon 2 of the CYP2R1 gene on chromosome 11p15.2. The inherited mutation caused the substitution of a proline for an evolutionarily conserved leucine at amino acid 99 in the CYP2R1 protein and eliminated vitamin D 25-hydroxylase enzyme activity. These data identify CYP2R1 as a biologically relevant vitamin D 25-hydroxylase and reveal the molecular basis of a human genetic disease, selective 25-hydroxyvitamin D deficiency.
Figures
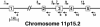
Similar articles
-
De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase.J Biol Chem. 2003 Sep 26;278(39):38084-93. doi: 10.1074/jbc.M307028200. Epub 2003 Jul 16. J Biol Chem. 2003. PMID: 12867411 Free PMC article.
-
Mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency.J Clin Endocrinol Metab. 2012 Oct;97(10):E2022-5. doi: 10.1210/jc.2012-1340. Epub 2012 Aug 1. J Clin Endocrinol Metab. 2012. PMID: 22855339 Free PMC article.
-
Vitamin D hydroxylases CYP2R1, CYP27B1 and CYP24A1 in renal cell carcinoma.Eur J Clin Invest. 2013 Dec;43(12):1282-90. doi: 10.1111/eci.12176. Epub 2013 Oct 12. Eur J Clin Invest. 2013. PMID: 24245571
-
CYP2R1 mutations causing vitamin D-deficiency rickets.J Steroid Biochem Mol Biol. 2017 Oct;173:333-336. doi: 10.1016/j.jsbmb.2016.07.014. Epub 2016 Jul 27. J Steroid Biochem Mol Biol. 2017. PMID: 27473561 Review.
-
Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form (review).Int J Mol Med. 2001 Feb;7(2):201-9. doi: 10.3892/ijmm.7.2.201. Int J Mol Med. 2001. PMID: 11172626 Review.
Cited by
-
CYP2R1 Mutations Impair Generation of 25-hydroxyvitamin D and Cause an Atypical Form of Vitamin D Deficiency.J Clin Endocrinol Metab. 2015 Jul;100(7):E1005-13. doi: 10.1210/jc.2015-1746. Epub 2015 May 5. J Clin Endocrinol Metab. 2015. PMID: 25942481 Free PMC article.
-
Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway.Respir Res. 2006 Apr 6;7(1):60. doi: 10.1186/1465-9921-7-60. Respir Res. 2006. PMID: 16600026 Free PMC article.
-
Research Trends of Vitamin D Metabolism Gene Polymorphisms Based on a Bibliometric Investigation.Genes (Basel). 2023 Jan 14;14(1):215. doi: 10.3390/genes14010215. Genes (Basel). 2023. PMID: 36672957 Free PMC article.
-
Novel Homozygous CYP27B1 Gene Mutation in Vitamin D-Dependent Rickets Type 1A (VDDR1A) Disorder: A Case Report.Front Endocrinol (Lausanne). 2022 May 18;13:862022. doi: 10.3389/fendo.2022.862022. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35663328 Free PMC article.
-
Vitamin D Deficiency During Pregnancy and Autism Spectrum Disorders Development.Front Psychiatry. 2020 Jan 31;10:987. doi: 10.3389/fpsyt.2019.00987. eCollection 2019. Front Psychiatry. 2020. PMID: 32082196 Free PMC article. Review.
References
-
- Jones, G., Strugnell, S. A. & DeLuca, H. F. (1998) Physiol. Rev. 78, 1193-1231. - PubMed
-
- Gupta, R. P., Hollis, B. W., Patel, S. B., Patrick, K. S. & Bell, N. H. (2004) J. Bone Miner. Res. 19, 680-688. - PubMed
-
- Yamasaki, T., Izumi, S., Ide, H. & Ohyama, Y. (March 16, 2004) J. Biol. Chem., 10.1074/jbc.M312601200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

