SecB is a bona fide generalized chaperone in Escherichia coli
- PMID: 15128935
- PMCID: PMC419649
- DOI: 10.1073/pnas.0402398101
SecB is a bona fide generalized chaperone in Escherichia coli
Abstract
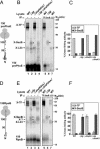
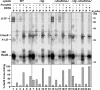
It is known that the DnaK and Trigger Factor (TF) chaperones cooperate in the folding of newly synthesized cytosolic proteins in Escherichia coli. We recently showed that despite a very narrow temperature range of growth and high levels of aggregated cytosolic proteins, E. coli can tolerate deletion of both chaperones, suggesting that other chaperones might be involved in this process. Here, we show that the secretion-dedicated chaperone SecB efficiently suppresses both the temperature sensitivity and the aggregation-prone phenotypes of a strain lacking both TF and DnaK. SecB suppression is independent of a productive interaction with the SecA subunit of the translocon. Furthermore, in vitro cross-linking experiments demonstrate that SecB can interact both co- and posttranslationally with short nascent chains of both secretory and cytosolic proteins. Finally, we show that such cotranslational substrate recognition by SecB is greatly suppressed in the presence of ribosome-bound TF, but not by DnaK. Taken together, our data demonstrate that SecB acts as a bona fide generalized chaperone.
Figures


References
-
- Hartl, F. U. & Hayer-Hartl, M. (2002) Science 295, 1852-1858. - PubMed
-
- Bukau, B., Deuerling, E., Pfund, C. & Craig, E. A. (2000) Cell 101, 119-122. - PubMed
-
- Deuerling, E., Schulze-Specking, A., Tomoyasu, T., Mogk, A. & Bukau, B. (1999) Nature 400, 693-696. - PubMed
-
- Teter, S. A., Houry, W. A., Ang, D., Tradler, T., Rockabrand, D., Fischer, G., Blum, P., Georgopoulos, C. & Hartl, F. U. (1999) Cell 97, 755-765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

