An invariant aspartic acid in the DNA glycosylase domain of DEMETER is necessary for transcriptional activation of the imprinted MEDEA gene
- PMID: 15128940
- PMCID: PMC409944
- DOI: 10.1073/pnas.0402328101
An invariant aspartic acid in the DNA glycosylase domain of DEMETER is necessary for transcriptional activation of the imprinted MEDEA gene
Abstract
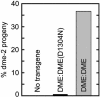
Helix-hairpin-helix DNA glycosylases are typically small proteins that initiate repair of DNA by excising damaged or mispaired bases. An invariant aspartic acid in the active site is involved in catalyzing the excision reaction. Replacement of this critical residue with an asparagine severely reduces catalytic activity but preserves enzyme stability and structure. The Arabidopsis DEMETER (DME) gene encodes a large 1,729-aa polypeptide with a 200-aa DNA glycosylase domain. DME is expressed primarily in the central cell of the female gametophyte. DME activates maternal allele expression of the imprinted MEDEA (MEA) gene in the central cell and is required for seed viability. We mutated the invariant aspartic acid at position 1304 in DME to asparagine (D1304N) to determine whether the catalytic activity of the DNA glycosylase domain is required for DME function in vivo. Transgenes expressing wild-type DME in the central cell rescue seed abortion caused by a mutation in the endogenous DME gene and activate maternal MEA:GFP transcription. However, transgenes expressing the D1304N mutant DME do not rescue seed abortion or activate maternal MEA:GFP transcription. Whereas ectopic expression of the wild-type DME polypeptide in pollen is sufficient to activate ectopic paternal MEA and MEA:GUS expression, equivalent expression of the D1304N mutant DME in pollen failed to do so. These results show that the conserved aspartic acid residue is necessary for DME to function in vivo and suggest that an active DNA glycosylase domain, normally associated with DNA repair, promotes gene transcription that is essential for gene imprinting.
Figures




Similar articles
-
DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation.Cell. 2006 Feb 10;124(3):495-506. doi: 10.1016/j.cell.2005.12.034. Cell. 2006. PMID: 16469697 Free PMC article.
-
DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in arabidopsis.Cell. 2002 Jul 12;110(1):33-42. doi: 10.1016/s0092-8674(02)00807-3. Cell. 2002. PMID: 12150995
-
Imprinting of the MEA Polycomb gene is controlled by antagonism between MET1 methyltransferase and DME glycosylase.Dev Cell. 2003 Dec;5(6):891-901. doi: 10.1016/s1534-5807(03)00361-7. Dev Cell. 2003. PMID: 14667411
-
Imprinting in plants and its underlying mechanisms.J Genet Genomics. 2013 May 20;40(5):239-47. doi: 10.1016/j.jgg.2013.04.003. Epub 2013 Apr 20. J Genet Genomics. 2013. PMID: 23706299 Review.
-
Preventing transcriptional gene silencing by active DNA demethylation.FEBS Lett. 2005 Oct 31;579(26):5889-98. doi: 10.1016/j.febslet.2005.08.039. Epub 2005 Aug 31. FEBS Lett. 2005. PMID: 16162337 Review.
Cited by
-
Role of Base Excision "Repair" Enzymes in Erasing Epigenetic Marks from DNA.Chem Rev. 2016 Oct 26;116(20):12711-12729. doi: 10.1021/acs.chemrev.6b00191. Epub 2016 Aug 8. Chem Rev. 2016. PMID: 27501078 Free PMC article. Review.
-
DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation.Cell. 2006 Feb 10;124(3):495-506. doi: 10.1016/j.cell.2005.12.034. Cell. 2006. PMID: 16469697 Free PMC article.
-
Comparative survey of plastid and mitochondrial targeting properties of transcription factors in Arabidopsis and rice.Mol Genet Genomics. 2007 Jun;277(6):631-46. doi: 10.1007/s00438-007-0214-4. Epub 2007 Feb 13. Mol Genet Genomics. 2007. PMID: 17295027
-
Characterization of Imprinted Genes in Rice Reveals Conservation of Regulation and Imprinting with Other Plant Species.Plant Physiol. 2018 Aug;177(4):1754-1771. doi: 10.1104/pp.17.01621. Epub 2018 Jun 18. Plant Physiol. 2018. PMID: 29914891 Free PMC article.
-
Histone H1 affects gene imprinting and DNA methylation in Arabidopsis.Plant J. 2012 Sep;71(5):776-86. doi: 10.1111/j.1365-313X.2012.05028.x. Epub 2012 Jun 14. Plant J. 2012. PMID: 22519754 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

