Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion
- PMID: 15128946
- PMCID: PMC419674
- DOI: 10.1073/pnas.0401528101
Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion
Abstract
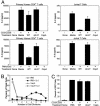
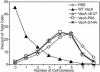
Recent evidence indicates that the secreted Helicobacter pylori vacuolating toxin (VacA) inhibits the activation of T cells. VacA blocks IL-2 secretion in transformed T cell lines by suppressing the activation of nuclear factor of activated T cells (NFAT). In this study, we investigated the effects of VacA on primary human CD4(+) T cells. VacA inhibited the proliferation of primary human T cells activated through the T cell receptor (TCR) and CD28. VacA-treated Jurkat T cells secreted markedly diminished levels of IL-2 compared with untreated cells, whereas VacA-treated primary human T cells continued to secrete high levels of IL-2. Further experiments indicated that the VacA-induced inhibition of primary human T cell proliferation was not attributable to VacA effects on NFAT activation or IL-2 secretion. We show here that VacA suppresses IL-2-induced cell-cycle progression and proliferation of primary human T cells without affecting IL-2-dependent survival. Through the analysis of a panel of mutant VacA proteins, we demonstrate that VacA-mediated inhibition of T cell proliferation requires an intact N-terminal hydrophobic region necessary for the formation of anion-selective membrane channels. Remarkably, we demonstrate that one of these mutant VacA proteins [VacA-Delta(6-27)] abrogates the immunosuppressive actions of wild-type VacA in a dominant-negative fashion. We suggest that VacA may inhibit the clonal expansion of T cells that have already been activated by H. pylori antigens, thereby allowing H. pylori to evade the adaptive immune response and establish chronic infection.
Figures


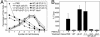
Similar articles
-
Resistance of primary murine CD4+ T cells to Helicobacter pylori vacuolating cytotoxin.Infect Immun. 2007 Jan;75(1):334-41. doi: 10.1128/IAI.01063-06. Epub 2006 Oct 30. Infect Immun. 2007. PMID: 17074854 Free PMC article.
-
PKC-dependent endocytosis of the Helicobacter pylori vacuolating cytotoxin in primary T lymphocytes.Cell Microbiol. 2011 Mar;13(3):482-96. doi: 10.1111/j.1462-5822.2010.01551.x. Epub 2010 Dec 13. Cell Microbiol. 2011. PMID: 21083636
-
Integrin subunit CD18 Is the T-lymphocyte receptor for the Helicobacter pylori vacuolating cytotoxin.Cell Host Microbe. 2008 Jan 17;3(1):20-9. doi: 10.1016/j.chom.2007.11.003. Cell Host Microbe. 2008. PMID: 18191791
-
Pleiotropic actions of Helicobacter pylori vacuolating cytotoxin, VacA.Tohoku J Exp Med. 2010 Jan;220(1):3-14. doi: 10.1620/tjem.220.3. Tohoku J Exp Med. 2010. PMID: 20046046 Review.
-
Vacuolating cytotoxin A (VacA) - A multi-talented pore-forming toxin from Helicobacter pylori.Toxicon. 2016 Aug;118:27-35. doi: 10.1016/j.toxicon.2016.04.037. Epub 2016 Apr 20. Toxicon. 2016. PMID: 27105670 Review.
Cited by
-
A Helicobacter pylori vacuolating toxin mutant that fails to oligomerize has a dominant negative phenotype.Infect Immun. 2006 Mar;74(3):1786-94. doi: 10.1128/IAI.74.3.1786-1794.2006. Infect Immun. 2006. PMID: 16495552 Free PMC article.
-
Analysis of a beta-helical region in the p55 domain of Helicobacter pylori vacuolating toxin.BMC Microbiol. 2010 Feb 23;10:60. doi: 10.1186/1471-2180-10-60. BMC Microbiol. 2010. PMID: 20178613 Free PMC article.
-
Structural modifications of Helicobacter pylori lipopolysaccharide: an idea for how to live in peace.World J Gastroenterol. 2014 Aug 7;20(29):9882-97. doi: 10.3748/wjg.v20.i29.9882. World J Gastroenterol. 2014. PMID: 25110419 Free PMC article. Review.
-
The intermediate region of Helicobacter pylori VacA is a determinant of toxin potency in a Jurkat T cell assay.Infect Immun. 2012 Aug;80(8):2578-88. doi: 10.1128/IAI.00052-12. Epub 2012 May 14. Infect Immun. 2012. PMID: 22585965 Free PMC article.
-
Reconstitution of Helicobacter pylori VacA toxin from purified components.Biochemistry. 2010 Jul 13;49(27):5743-52. doi: 10.1021/bi100618g. Biochemistry. 2010. PMID: 20527875 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

