DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response
- PMID: 15129672
- PMCID: PMC1782456
- DOI: 10.1111/j.1365-2567.2004.01823.x
DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response
Abstract
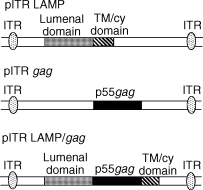
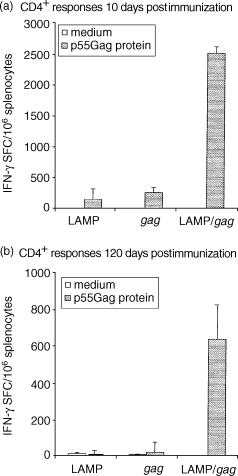
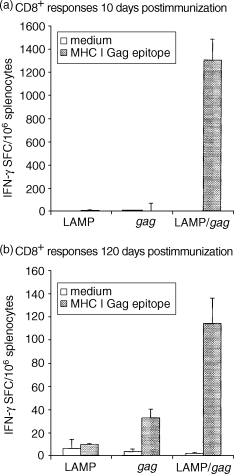
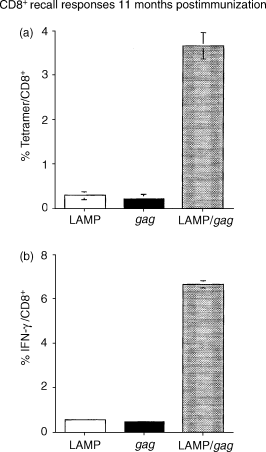
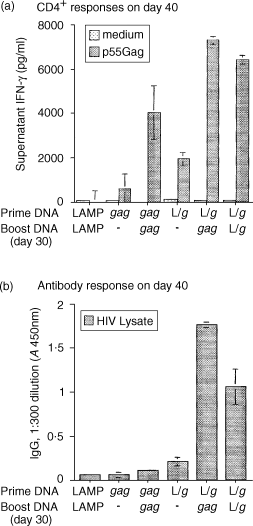
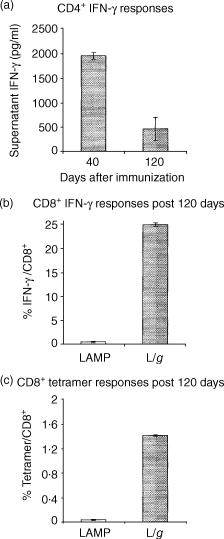
Antigen presentation by major histocompatibility complex type II (MHC II) molecules and activation of CD4+ helper T cells are critical for the generation of immunological memory. We previously described a DNA vaccine encoding human immunodeficiency virus-1 p55Gag as a chimera with the lysosome-associated membrane protein (LAMP/gag). The LAMP/gag chimera protein traffics to the MHC II compartment of transfected cells and elicits enhanced immune responses as compared to a DNA vaccine encoding native gag not targeted to the MHC II compartment. We have now investigated the long-term responses of immunized mice and show that the LAMP/gag DNA vaccine promotes long-lasting B cell- and CD4+ and CD8+ T-cell memory responses induced by DNA encoding non-targeted Gag decay rapidly and elicit very low or undetectable levels of gag DNA is sufficient to generate T-cell memory. Following this initial priming immunization with LAMP/gag DNA, booster immunizations with native gag DNA or the LAMP/gag chimera are equally efficient in eliciting B- and T-cell secondary responses, results in accordance with observations that secondary expansion of CD8+ cells in the boost phase does not require additional CD4+ help. These findings underscore the significance of targeting DNA-encoded vaccine antigens to the MHC II processing compartments for induction of long-term immunological memory.
Figures
Similar articles
-
HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses.J Biol Chem. 2003 Sep 26;278(39):37926-36. doi: 10.1074/jbc.M303336200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824194
-
DNA encoding an HIV-1 Gag/human lysosome-associated membrane protein-1 chimera elicits a broad cellular and humoral immune response in Rhesus macaques.PLoS One. 2006 Dec 27;1(1):e135. doi: 10.1371/journal.pone.0000135. PLoS One. 2006. PMID: 17205139 Free PMC article.
-
Mucosal and systemic anti-GAG immunity induced by neonatal immunization with HIV LAMP/gag DNA vaccine in mice.Immunobiology. 2011 Apr;216(4):505-12. doi: 10.1016/j.imbio.2010.08.007. Epub 2010 Sep 25. Immunobiology. 2011. PMID: 20870310
-
Inverted terminal repeat sequences of adeno-associated virus enhance the antibody and CD8(+) responses to a HIV-1 p55Gag/LAMP DNA vaccine chimera.Virology. 2004 Jun 1;323(2):220-32. doi: 10.1016/j.virol.2004.02.025. Virology. 2004. PMID: 15193918
-
Immunization of neonatal mice with LAMP/p55 HIV gag DNA elicits robust immune responses that last to adulthood.Virology. 2010 Oct 10;406(1):37-47. doi: 10.1016/j.virol.2010.06.050. Epub 2010 Jul 29. Virology. 2010. PMID: 20667577
Cited by
-
Modifying the HIV-1 env gp160 gene to improve pDNA vaccine-elicited cell-mediated immune responses.Vaccine. 2008 Sep 19;26(40):5083-94. doi: 10.1016/j.vaccine.2008.03.092. Epub 2008 Apr 24. Vaccine. 2008. PMID: 18485543 Free PMC article.
-
Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease.Arch Neurol. 2004 Dec;61(12):1859-64. doi: 10.1001/archneur.61.12.1859. Arch Neurol. 2004. PMID: 15596606 Free PMC article.
-
A DNA vaccine against yellow fever virus: development and evaluation.PLoS Negl Trop Dis. 2015 Apr 13;9(4):e0003693. doi: 10.1371/journal.pntd.0003693. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25875109 Free PMC article.
-
Cyclophilin A as a potential genetic adjuvant to improve HIV-1 Gag DNA vaccine immunogenicity by eliciting broad and long-term Gag-specific cellular immunity in mice.Hum Vaccin Immunother. 2016;12(2):545-53. doi: 10.1080/21645515.2015.1082692. Hum Vaccin Immunother. 2016. PMID: 26305669 Free PMC article.
-
The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells.J Clin Invest. 2008 Apr;118(4):1427-36. doi: 10.1172/JCI34224. J Clin Invest. 2008. PMID: 18324335 Free PMC article.
References
-
- Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–46. - PubMed
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. - PubMed
-
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. - PubMed
-
- Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–63. - PubMed
-
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
