Anatomical features of anti-viral immunity in the respiratory tract
- PMID: 15130500
- PMCID: PMC7128764
- DOI: 10.1016/j.smim.2004.02.003
Anatomical features of anti-viral immunity in the respiratory tract
Abstract
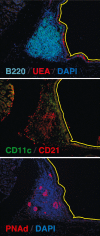
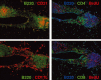
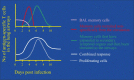
The mucosal surfaces of the lungs are a major portal of entry for virus infections and there are urgent needs for new vaccines that promote effective pulmonary immunity. However, we have only a rudimentary understanding of the requirements for effective cellular immunity in the respiratory tract. Recent studies have revealed that specialized cellular immune responses and lymphoid tissues are involved in the protection of distinct anatomical microenvironments of the respiratory tract, such as the large airways of the nose and the alveolar airspaces. This review discusses some of the anatomical features of anti-viral immunity in the respiratory tract including the role of local lymphoid tissues and the relationship between effector and memory T cells in the airways, the lung parenchyma, and lymphoid organs.
Figures
References
-
- Nguyen-Van-Tam J.S., Hampson A.W. The epidemiology and clinical impact of pandemic influenza. Vaccine. 2003;21:1762–1768. - PubMed
-
- Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. - PubMed
-
- Woodland D.L., Hogan R.J., Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol. Res. 2001;24:53–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

