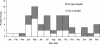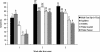Evaluation of rapid diagnostic tests for typhoid fever
- PMID: 15131144
- PMCID: PMC404619
- DOI: 10.1128/JCM.42.5.1885-1889.2004
Evaluation of rapid diagnostic tests for typhoid fever
Abstract
Laboratory diagnosis of typhoid fever requires isolation and identification of Salmonella enterica serotype Typhi. In many areas where this disease is endemic, laboratory capability is limited. Recent advances in molecular immunology have led to the identification of sensitive and specific markers for typhoid fever and technology to manufacture practical and inexpensive kits for their rapid detection. We evaluated three commercial kits for serologic diagnosis of typhoid fever. Patients presenting with > or = 4 days of fever were enrolled at two hospitals in Southern Vietnam. Cases were patients with serotype Typhi isolated from blood samples, and controls were patients with other laboratory-confirmed illnesses. Serotype Typhi isolates were confirmed and tested for antimicrobial susceptibility at the Pasteur Institute in Ho Chi Minh City. The Widal test was run at the hospitals and the Pasteur Institute. Sera were shipped frozen to the Centers for Disease Control and Prevention and tested by using Multi-Test Dip-S-Ticks, TyphiDot, and TUBEX to detect immunoglobulin G (IgG), IgG and IgM, and IgM, respectively. Package insert protocol instructions were followed. We enrolled 59 patients and 21 controls. The sensitivity and specificity findings were as follows: 89 and 53% for Multi-Test Dip-S-Ticks, 79 and 89% for TyphiDot, 78 and 89% for TUBEX, and 64 and 76% for Widal testing in hospitals and 61% and 100% for Widal testing at the Pasteur Institute. For all assays, the sensitivity was highest in the second week of illness. The Widal test was insensitive and displayed interoperator variability. Two rapid kits, TyphiDot and TUBEX, demonstrated promising results.
Figures
References
-
- Bhutta, Z. A., and N. Mansurali. 1999. Rapid serologic diagnosis of pediatric typhoid fever in an endemic area: a prospective comparative evaluation of two dot-enzyme immunoassays and the Widal test. Am. J. Trop. Med. Hyg. 61:654-657. - PubMed
-
- Fadeel, M. A., J. A. Crump, F. J. Mahoney, I. A. Nakhla, A. M. Mansour, B. Reyad, D. E. Melegi, Y. Sultan, E. D. Mintz, and W. F. Bibb. 2004. Rapid diagnosis of typhoid fever by enzyme-linked immunosorbent assay detection of Salmonella serotype Typhi antigens in urine. Am. J. Trop. Med. Hyg. 70:323-328. - PubMed
-
- Gilman, R. H., M. Terminel, M. M. Levine, P. Hernandez-Mendoza, and R. B. Hornick. 1975. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet i:1211-1213. - PubMed
-
- Gopalakrishnan, V., W. Y. Sekhar, E. H. Soo, and S. Devi. 2002. Typhoid fever in Kuala Lumpur and a comparative evaluation of two commercial diagnostic kits for detection of antibodies to Salmonella typhi. Singapore Med. J. 43:354-358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources