Amplification of coccidioidal DNA in clinical specimens by PCR
- PMID: 15131158
- PMCID: PMC404645
- DOI: 10.1128/JCM.42.5.1982-1985.2004
Amplification of coccidioidal DNA in clinical specimens by PCR
Abstract
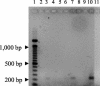
Coccidioides DNA was amplified from serum by a PCR using coccidioid-specific primers. A 239-bp product was visualized when 10 fg of exogenous coccidioidal DNA was subjected to amplification. This product was demonstrated in some human and mouse sera prior to the detection of coccidioidal antibodies.
Figures

Comment in
-
Amplification of coccidioidal DNA in clinical specimens by PCR.J Clin Microbiol. 2005 Mar;43(3):1492; author reply 1492-3. doi: 10.1128/JCM.43.3.1492-1493.2005. J Clin Microbiol. 2005. PMID: 15750145 Free PMC article. No abstract available.
References
-
- Clark, K. A., and D. McAllister. 1996. Direct detection of Coccidioides immitis in clinical specimens using target amplification, p. 129-136. In H. E. Einstein and A. Cantanzaro (ed.), Coccidioidomycosis. Proceedings of the 5th International Conference. National Foundation for Infectious Diseases, Bethesda, Md.
-
- Fisher, M. C., G. L. Koenig, T. J. White, and J. W. Taylor. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94:73-84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

