The control of storage xyloglucan mobilization in cotyledons of Hymenaea courbaril
- PMID: 15133152
- PMCID: PMC429377
- DOI: 10.1104/pp.104.040220
The control of storage xyloglucan mobilization in cotyledons of Hymenaea courbaril
Abstract
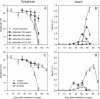
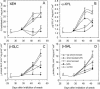
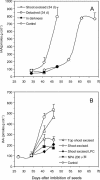
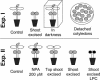
Hymenaea courbaril is a leguminous tree species from the neotropical rain forests. Its cotyledons are largely enriched with a storage cell wall polysaccharide (xyloglucan). Studies of cell wall storage polymers have been focused mostly on the mechanisms of their disassembly, whereas the control of their mobilization and the relationship between their metabolism and seedling development is not well understood. Here, we show that xyloglucan mobilization is strictly controlled by the development of first leaves of the seedling, with the start of its degradation occurring after the beginning of eophyll (first leaves) expansion. During the period of storage mobilization, an increase in the levels of xyloglucan hydrolases, starch, and free sugars were observed in the cotyledons. Xyloglucan mobilization was inhibited by shoot excision, darkness, and by treatment with the auxin-transport inhibitor N-1-naphthylphthalamic acid. Analyses of endogenous indole-3-acetic acid in the cotyledons revealed that its increase in concentration is followed by the rise in xyloglucan hydrolase activities, indicating that auxin is directly related to xyloglucan mobilization. Cotyledons detached during xyloglucan mobilization and treated with 2,4-dichlorophenoxyacetic acid showed a similar mobilization rate as in attached cotyledons. This hormonal control is probably essential for the ecophysiological performance of this species in their natural environment since it is the main factor responsible for promoting synchronism between shoot growth and reserve degradation. This is likely to increase the efficiency of carbon reserves utilization by the growing seedling in the understorey light conditions of the rain forest.
Figures



References
-
- Alcântara PHN (2000) Isolamento e caracterização das enzimas xiloglucano endotransglicosilase e β-galactosidase do catabolismo do xiloglucano de reserva dos cotilédones de Hymenaea courbaril L. (Leguminosae-Caesalpinioideae). PhD thesis. Federal University of São Paulo (UNIFESP), São Paulo, Brazil
-
- Alcântara PHN, Dietrich SMC, Buckeridge MS (1999) Xyloglucan mobilisation and purification of a (XLLG/XLXG) specific β-galactosidase from cotyledons of Copaifera langsdorffii. Plant Physiol Biochem 37: 653–663
-
- Arêas JAG, Lajolo FM (1980) Determinação enzimática específica de amido, glicose, frutose e sacarose em bananas pré-climatéricas e climatéricas. An Farm Quím S Paulo 20: 307–318 - PubMed
-
- Bartel B (1997) Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 51–66 - PubMed
-
- Bewley JD, Black M (1994) Seeds. Physiology of Development and Germination. Ed 2. Plenum Press, New York
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

