Accessibility of introduced cysteines in chemoreceptor transmembrane helices reveals boundaries interior to bracketing charged residues
- PMID: 15133159
- PMCID: PMC2279978
- DOI: 10.1110/ps.04648604
Accessibility of introduced cysteines in chemoreceptor transmembrane helices reveals boundaries interior to bracketing charged residues
Abstract
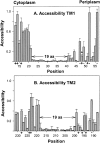
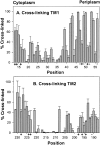
Two hydrophobic sequences, 24 and 30 residues long, identify the membrane-spanning segments of chemoreceptor Trg from Escherichia coli. As in other related chemoreceptors, these helical sequences are longer than the minimum necessary for an alpha-helix to span the hydrocarbon region of a biological membrane. Thus, the specific positioning of the segments relative to the hydrophobic part of the membrane cannot be deduced from sequence alone. With the aim of defining the positioning for Trg experimentally, we determined accessibility of a hydrophilic sulfhydryl reagent to cysteines introduced at each position within and immediately outside the two hydrophobic sequences. For both sequences, there was a specific region of uniformly low accessibility, bracketed by regions of substantial accessibility. The two low-accessibility regions were each 19 residues long and were in register in the three-dimensional organization of the transmembrane domain deduced from independent data. None of the four hydrophobic-hydrophilic boundaries for these two membrane-embedded sequences occurred at a charged residue. Instead, they were displaced one to seven residues internal to the charged side chains bracketing the extended hydrophobic sequences. Many hydrophobic sequences, known or predicted to be membrane-spanning, are longer than the minimum necessary helical length, but precise membrane boundaries are known for very few. The cysteine-accessibility approach provides an experimental strategy for determining those boundaries that could be widely applicable.
Figures



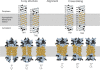

References
-
- Abramson, J., Smirnova, I., Kasho, V., Verner, G., Kaback, H.R., and Iwata, S. 2003. Structure and mechanism of the lactose permease of Escherichia coli. Science 301 610–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

