The antihypertensive efficacy and safety of a chronotherapeutic formulation of propranolol in patients with hypertension
- PMID: 15133405
- PMCID: PMC8109330
- DOI: 10.1111/j.1076-7460.2004.3624.x
The antihypertensive efficacy and safety of a chronotherapeutic formulation of propranolol in patients with hypertension
Abstract
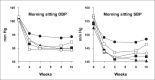
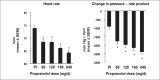
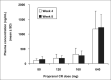
This study evaluated the antihypertensive efficacy and tolerability of a chronotherapeutic formulation of propranolol designed for nighttime dosing (propranolol controlled release [CR]). A total of 434 patients with mild-to-moderate hypertension were randomized to placebo or to one of four doses of propranolol CR (80, 120, 160, or 640 mg/d). At baseline, the mean morning blood pressures were similar in each treatment group and averaged 152/101 mm Hg. After 8 weeks of treatment, morning diastolic blood pressure, the primary efficacy measurement, was significantly reduced from baseline in placebo (-6.98 mm Hg) and all propranolol groups (p<0.001). The decreases ranged from 10.1 mm Hg in the 80-mg/d group to 11.0 mm Hg in the 120-mg/d group and were significantly larger than placebo in the 120-, 160-, and 640-mg/d groups (p<0.05). Blood pressure measured in the evening (trough) demonstrated similar antihypertensive efficacy. Heart rate and rate-pressure product were reduced in a dose-related manner by propranolol CR. The formulation was well tolerated with only fatigue and dizziness being reported more frequently than in the placebo group. Propranolol CR is an effective antihypertensive formulation that may reduce blood pressure during the morning period of maximum cardiovascular risk.
Figures

References
-
- Morning peak in the incidence of myocardial infarction: experience in the ISIS‐2 trial. Eur Heart J. 1992;13:594–598. - PubMed
-
- Cohen MC, Rohtla KM, Lavery CE, et al. Meta‐analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. - PubMed
-
- Willich SN, Levy D, Rocco MB, et al. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. - PubMed
-
- Elliott WJ. Circadian variation in the timing of stroke onset: a meta‐analysis. Stroke. 1998;29:992–996. - PubMed
-
- Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

